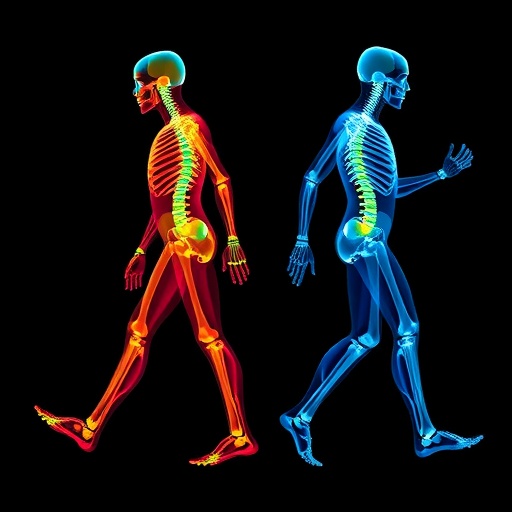
In the evolving landscape of neurological healthcare, gait assessment stands as a cornerstone diagnostic and monitoring tool, offering critical insights into patient conditions ranging from cerebral palsy to Parkinson’s disease. Traditionally, however, such assessments have relied heavily on subjective clinical observations, which are qualitative and susceptible to observer bias. The inherent limitations of these methods have catalyzed a push towards more quantitative, scalable, and objective solutions. Recent advancements in artificial intelligence (AI), coupled with the ubiquity of smartphones equipped with sophisticated cameras, have opened new horizons for gait analysis. Despite these technological strides, a fundamental roadblock persists: the scarcity of comprehensive, diverse clinical datasets necessary to train robust AI models that can generalize across varied populations and sensor environments. This scarcity, often rooted in stringent privacy regulations and the logistical complexities of data collection, has confined most existing gait analysis algorithms to niche applications, limiting their clinical impact.
Addressing this critical bottleneck, a multidisciplinary team of researchers from IBM Research, the Cleveland Clinic, and the University of Tsukuba has unveiled a groundbreaking framework that harnesses generative AI to produce synthetic gait data. Their methodology diverges fundamentally from typical data augmentation techniques by embedding physics-based musculoskeletal simulations within the generative process. These simulations meticulously capture a spectrum of biomechanical parameters that reflect real-world heterogeneity: age-dependent musculoskeletal variations, pathological gait patterns, and the influence of different sensor configurations. By integrating this bio-physical realism with AI’s synthetic data generation capacity, the researchers have crafted a rich and diverse dataset that transcends conventional limitations and equips evolving AI models with the capacity to perform reliably across a multitude of clinical contexts.
Central to their approach is the deployment of physics-based musculoskeletal modeling, which simulates the dynamic interaction of bones, muscles, and joints during gait cycles. This mechanistic foundation ensures that generated synthetic data maintain physiological authenticity, accurately mirroring the nuances of human movement under varying health conditions. By encompassing patients as diverse as children with cerebral palsy and adults afflicted by neurodegenerative diseases, alongside healthy controls, the simulations capture a broad pathological spectrum. Moreover, by varying sensor parameters—such as camera angle and resolution in smartphone video captures—the synthetic dataset reflects real-world heterogeneity in data acquisition, enhancing the generalizability of subsequent AI models.
.adsslot_nHhQT2vB9R{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_nHhQT2vB9R{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_nHhQT2vB9R{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
The team rigorously validated their framework against an extensive, real-world gait dataset comprising over 12,000 recordings from more than 1,200 individuals. This cohort included patients with cerebral palsy, Parkinson’s disease, dementia, and other neurological disorders, providing a challenging testbed for model evaluation. Results from these validation studies unveiled two transformative capabilities. First, models exclusively pretrained on synthetic data demonstrated “zero-shot” performance comparable to, or in some cases surpassing, models trained on real-world datasets. This finding is particularly remarkable considering that these AI models could estimate clinically significant gait parameters—such as gait speed, step length, and temporal step dynamics—and infer muscle activation patterns from single-camera videos, showcasing the efficacy of synthetic data in capturing biomechanical complexity.
Beyond zero-shot learning, the framework exhibited exceptional data efficiency in transfer learning scenarios. By initially pretraining AI models on large-scale synthetic datasets, followed by fine-tuning with limited real-world data, these hybrid models outperformed state-of-the-art deep learning architectures trained solely on extensive real-world datasets. This novel two-step approach not only maximizes the utility of scarce clinical data, especially for rare conditions, but also circumvents privacy-related obstacles by reducing reliance on large-scale patient data collection. The efficiency gains promise to accelerate the deployment of robust gait analysis tools in clinical settings, catalyzing personalized disease monitoring and management.
The implications of these findings extend significantly into the management of neurological disorders. Accurate quantification of gait aberrations aids clinicians in disease detection, severity assessment, and therapy evaluation. By facilitating precise, objective, and scalable gait analysis using readily accessible smartphone videos, this AI-driven approach could democratize neurological monitoring, particularly benefiting underserved populations with limited access to specialized motion analysis laboratories. Such scalable solutions hold the potential to complement clinical workflows, enabling longitudinal tracking of disease progression with minimal patient burden.
Moreover, the integration of physics-based musculoskeletal simulation with generative AI represents a paradigm shift in synthetic data utilization. Unlike traditional synthetic datasets limited to simple pattern replication, these bio-realistic synthetic gaits serve as a high-fidelity substitute for real clinical data, preserving mechanistic plausibility and inter-subject variability. This innovation paves the way not only for gait analysis but also for broader healthcare applications where data scarcity and privacy issues hinder AI development. Disease-specific synthetic data generation might soon become a cornerstone for training reliable, equitable AI systems across diverse biomedical domains.
The researchers’ interdisciplinary collaboration underscores the necessity of combining expertise in computational biomechanics, machine learning, and clinical neurology. Their framework bridges these domains effectively, creating a translational pathway from theoretical simulations to practical clinical tools. This synergy ensures that AI models are not only technically sophisticated but also clinically relevant, thus fostering trust and adoption among healthcare professionals. Future expansions of this work could include integrating additional sensor modalities, such as inertial measurement units or electromyography, further enriching synthetic datasets to emulate multifaceted patient monitoring scenarios.
While the study chiefly focuses on neurological disorders, its principles may generalize across various musculoskeletal and mobility-related conditions. For example, synthetic musculoskeletal simulation could enable early detection of orthopedic impairments or rehabilitative progress post-injury. By providing a scalable platform for data generation, this approach could transform clinical research paradigms, reducing dependency on exhaustive patient recruitment and invasive instrumentation, thereby accelerating innovation cycles.
Ethical considerations remain paramount in clinical AI development. By leveraging synthetic data, the framework ameliorates privacy-related ethical challenges inherent to patient data usage. Synthetic datasets mitigate risks of patient re-identification and comply seamlessly with data governance frameworks, facilitating broader research collaborations and multi-institutional validations. This ethical advantage adds additional impetus for adopting synthetic data-driven methodologies in sensitive healthcare domains.
Looking ahead, the research team envisions integrating their synthetic data approach with real-time gait monitoring applications powered by ubiquitous mobile devices. Such convergence could usher in an era of continuous, passive health monitoring, empowering patients and clinicians with timely biomarker feedback. As AI models mature, their deployment could expand into telemedicine, rural healthcare, and personalized rehabilitation, substantially influencing public health outcomes.
Subject of Research: Development of synthetic musculoskeletal gait data for generalized and equitable AI-based clinical motion analysis.
Article Title: Utility of synthetic musculoskeletal gaits for generalizable healthcare applications
News Publication Date: July 4, 2025
Web References:
https://doi.org/10.1038/s41467-025-61292-1
References:
Arai, T., et al. (2025). Utility of synthetic musculoskeletal gaits for generalizable healthcare applications. Nature Communications. DOI: 10.1038/s41467-025-61292-1
Keywords:
Health care; Patient monitoring; Personalized medicine; Machine learning; Artificial intelligence; Neurological disorders; Computer simulation
Tags: advancements in neurological healthcareAI-driven healthcare innovationsartificial intelligence in neurologyclinical gait analysisgait analysis for Parkinson’s diseaseGenerative AI in healthcareinterdisciplinary research in gait analysismusculoskeletal simulation technologyobjective gait measurement techniquesovercoming data scarcity in healthcarequantitative gait assessment methodssynthetic data generation for clinical research