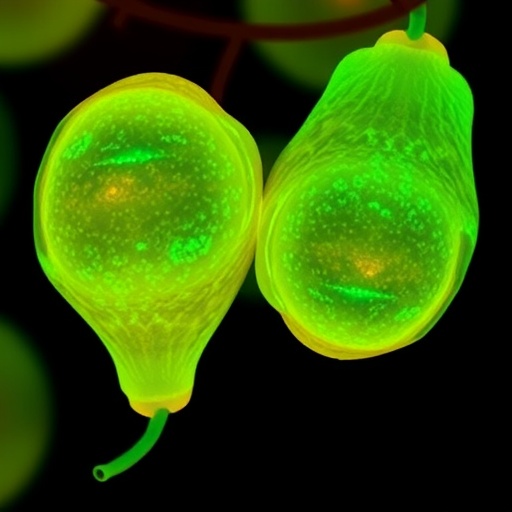
For the first time in botanical science, researchers have successfully visualized the precise spatiotemporal patterns of active lignin deposition within living pear cells. This unprecedented achievement enables the tracing of lignification as it unfolds in real time during fruit development, revealing how solitary cells embark on lignin synthesis as early as 10 days after full bloom (DAFB). This initiation triggers a cascade of lignification that culminates in the formation of substantial, rigid clusters of stone cells, which dramatically influence the texture and quality of pear fruit.
Pear fruit holds a valuable place in global agriculture due to its appealing flavor and nutritional benefits. However, its commercial value is often compromised by an undesirable gritty texture, a consequence of hardened, lignified stone cells embedded within the pulp. These stone cells, known as sclereids, are incredibly rich in lignin—a complex phenolic polymer integral to plant structural integrity. While lignin fortifies plants by reinforcing cell walls, its accumulation within edible fruit tissue undermines palatability and processing efficiency. Although prior genetic and biochemical investigations have shed light on certain regulatory components of pear lignification, they have fallen short of elucidating the fine-scale dynamics underlying the onset and spatial organization of lignified stone cells during fruit maturation.
Traditional histological and imaging methods have encountered fundamental limitations when attempting to differentiate newly synthesized lignin from the preexisting lignified matrix. This inability to temporally resolve lignin formation dynamics left a critical void in understanding the developmental biology of pear fruit at the cellular level. The recent study, led by Shaoling Zhang and colleagues at Nanjing Agricultural University and published in Plant Phenomics on February 25, 2025, overcomes these obstacles by applying a cutting-edge bioorthogonal chemical approach to chart lignification in vivo with high spatial and temporal precision, thereby constructing an intricate cellular atlas of stone cell development.
.adsslot_IcMRD0S9aT{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_IcMRD0S9aT{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_IcMRD0S9aT{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
Central to this breakthrough is the use of a synthetic lignin precursor termed 3-O-propargylcaffeyl alcohol (3-O-PCA), which chemically mimics the natural monolignol coniferyl alcohol but is uniquely functionalized with an alkyne moiety. This modification allows scientists to leverage click chemistry, specifically copper-catalyzed azide-alkyne cycloaddition (CuAAC), for the site-specific attachment of fluorescent tags after 3-O-PCA incorporation into newly forming lignin polymers. By administering 3-O-PCA to model plants such as Arabidopsis and pear seedlings, followed by labeling with Alexa 594-azide, the team detected vivid fluorescence at 561 nm emission, signifying successful integration of the probe into active lignification zones.
Initial validation in Arabidopsis showed robust lignin synthesis localized primarily within vascular bundles and interfascicular fibers, consistent with known lignification patterns. Extending the methodology to pear seedlings revealed strong fluorescence signals in xylem cells, parenchyma, and the phloem-associated stone cells, confirming that 3-O-PCA is metabolically incorporated and non-toxic, making it a powerful tool for in vivo visualization of lignin biosynthesis in perennial fruit crops.
By deploying this technological innovation across five distinct stages of pear fruit development, designated S1 through S5, the researchers unraveled the temporal progression of lignification. At stage S2, 10 DAFB, single parenchyma cells sporadically initiate lignin deposition. With advancing maturity into stage S3, lignification intensifies and expands into neighboring cells, forming nascent stone cell clusters. Stage S4 marks the enlargement of these clusters, while stage S5 exhibits fully matured stone cell aggregates with no further new lignin deposition, indicating cessation of lignification activity.
A particularly striking observation was the identification of primordial stone cells (PSCs) emerging at the early stages of lignification. These PSCs are distinguished by their larger size and unique morphological features relative to surrounding un-lignified parenchyma. Lignification radiates outward from PSCs in a radial, domino-like pattern, progressively converting adjacent cells into lignified sclereids. This spatial expansion is accompanied by the formation of characteristic pit canals and secondary cell walls, hallmarks of sclereid maturation.
Quantitative fluorescence analyses further elucidated the dynamics of lignin accumulation, revealing that cells at the periphery of clusters exhibit stronger fluorescent signals than central cells, suggestive of an active lignification front. This cascade mechanism offers vital insights into how lignified stone cell clusters aggregate and grow, reshaping our cellular understanding of fruit texture development.
The implications of this research extend beyond academic interest, offering a direct pathway for improving pear fruit quality through genetic and agronomic interventions aimed at modulating stone cell formation. By pinpointing the early developmental triggers and the mode of lignification expansion, breeders and growers can adopt targeted strategies to limit stone cell proliferation, thereby enhancing fruit texture and consumer appeal.
Moreover, the introduced bioorthogonal labeling strategy paves the way for advanced studies on cell wall biosynthesis and remodeling in a range of fruits and crops. Unlike conventional staining methods or microscopy, which fall short in temporal resolution and specificity, this approach delivers real-time visualization with both spatial and temporal fidelity, opening new horizons for plant developmental biology and crop science.
Future studies building upon this foundation may explore the genetic regulatory networks orchestrating PSC differentiation and lignin biosynthetic pathways during fruit maturation. Unraveling the molecular cues that govern the observed domino effect could enable precise engineering of lignification patterns, with significant ramifications for postharvest fruit quality and shelf life.
Ultimately, this pioneering in vivo tracing paradigm heralds a new era of precision plant phenomics, wherein metabolic processes like lignification can be dynamically monitored within living tissues. Such insights are invaluable for accelerating the development of superior fruit cultivars with optimized texture, nutritional profiles, and processing characteristics, aligning with global agricultural sustainability objectives.
In conclusion, the application of clickable synthetic monolignols for live-cell imaging represents a paradigm shift in our capacity to map complex developmental processes within fruit biology. The detailed cellular trajectory of stone cell lignification elucidated by Zhang and colleagues not only enhances fundamental understanding but also equips the agricultural community with potent tools to reshape fruit quality from the molecular to the macroscopic scale.
Subject of Research: Not applicable
Article Title: In vivo tracing the trajectory of cell lignification in pear fruit during development using click chemistry imaging
News Publication Date: 25-Feb-2025
Web References: http://dx.doi.org/10.1016/j.plaphe.2025.100010
References: 10.1016/j.plaphe.2025.100010
Keywords: Agriculture; Biochemistry; Technology
Tags: agricultural significance of pear lignificationchallenges in pear fruit processingdynamics of lignin depositionimpact of lignin on pear texturein vivo imaging of lignificationlignification patterns in botanical sciencelignin’s role in plant structural integritymechanisms of lignin synthesis in plantspear fruit quality and texturereal-time visualization of fruit developmentsclereids in pear fruitstone cell formation in pears