In a groundbreaking study published in Nature Communications, researchers have provided unprecedented insights into the immune system’s response to schistosome infection, focusing on the intricate behaviors of T cells during repeated controlled human exposures as opposed to natural infection environments. Schistosomiasis, a parasitic disease caused by schistosome worms, infects millions globally, posing significant public health challenges in endemic regions. Understanding the nuances of immune responses to this infection is essential for developing effective vaccines and therapeutic interventions.
The study meticulously compares the adaptive immune responses, particularly T cell dynamics, elicited by repeated controlled human infections with those arising from chronic, naturally acquired schistosome exposure. The experimental design employed a highly controlled infection model, allowing for precise measurement of T cell phenotypes and functions across multiple exposure events. This approach provided a rare window into how immunological memory and effector functions evolve in response to persistent antigen stimulation.
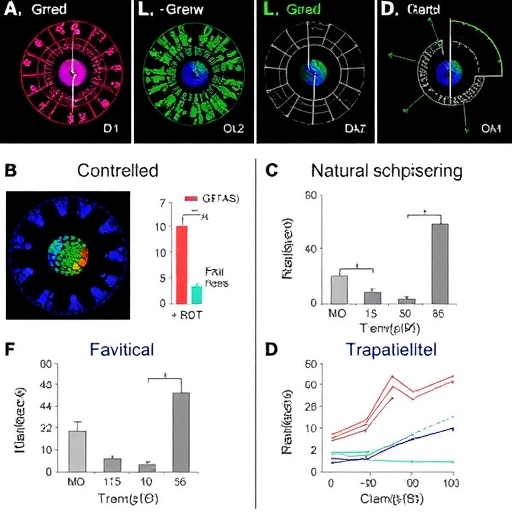
Central to the investigation were multiple subsets of T cells, including central memory, effector memory, and regulatory populations, each playing distinct roles in the host defense and immunopathology. Utilizing state-of-the-art flow cytometry and single-cell transcriptomics, the researchers delineated how repeated infections modulate the balance between protective immunity and immune regulation. Notably, the controlled model revealed that repeated schistosome exposure induces a potent, yet finely tuned, T cell response characterized by sustained activation without overt exhaustion.
.adsslot_IyzKTvejk5{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_IyzKTvejk5{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_IyzKTvejk5{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
This nuanced immune activation contrasts strikingly with the T cell profiles observed in individuals with natural, chronic infections, where prolonged antigen presence often leads to functional impairment or “exhaustion” of T cells. The study found that natural exposure tends to skew the immune landscape towards a more regulatory and less inflammatory milieu, arguably a survival adaptation that limits tissue damage while allowing parasite persistence.
Importantly, the research highlights the role of T helper 1 (Th1) and T helper 2 (Th2) cells in orchestrating the immune response. While Th2 responses have traditionally been linked to anti-schistosome immunity and granuloma formation around eggs, this study reveals that repeated controlled infections sustain a mixed Th1/Th2 environment. Such a balanced cytokine landscape may optimize parasite clearance while mitigating pathological consequences, an insight with direct implications for vaccine strategies aiming to mimic natural but protective immunity.
Delving deeper, the authors explore how regulatory T cells (Tregs) expand in response to continuous antigenic stimulation, potentially tempering the immune response to avoid excessive inflammation. However, excessive Treg activity in chronic natural infections may impede effective parasite clearance. The controlled infection model thus serves as a powerful system to dissect the thresholds at which immune modulation shifts from beneficial to detrimental, a crucial consideration for therapeutic targeting.
Beyond classical T cell subsets, the study also investigates the emerging roles of tissue-resident memory T cells (Trm), revealing their accumulation and retention in affected tissues upon repeated exposure. These cells may provide a localized, rapid response to reinfection, highlighting an underexplored axis of immunity in schistosomiasis. Their presence and functionality in humans, confirmed through detailed immunophenotyping, underscore the complexity and compartmentalization of the immune response in helminth infections.
The detailed kinetic analyses carried out demonstrate that each round of infection and treatment modulates T cell receptor (TCR) repertoires, suggesting that clonal selection and expansion processes evolve dynamically with repeated antigen encounters. This phenomenon not only shapes the quality of the immune response but also influences the breadth and durability of protective immunity, a critical factor for designing enduring vaccines.
Additionally, the role of co-inhibitory molecules such as PD-1 and CTLA-4 was examined, revealing their elevated expression in naturally infected individuals compared to the controlled infection group. These molecules typically mark cellular exhaustion states, providing an immunological checkpoint that may be co-opted by schistosomes to evade immune destruction. Modulating these pathways could enhance therapeutic efficacy, a promising avenue for future clinical studies.
The researchers also underscore the importance of applying controlled human infection models in parasitology, a field traditionally reliant on observational studies in endemic settings. By limiting variables and precisely timing infection events, this methodology allows robust conclusions about causality in immune regulation, setting a new standard for helminth immunology research.
Significantly, the study provides a rich dataset integrating immunophenotyping, transcriptomic profiling, and functional assays, offering a holistic view of T cell dynamics. This comprehensive approach reveals previously unrecognized populations and states of T cells that can now be targeted for improved immunomodulation in schistosomiasis. The implications extend beyond schistosomes, shedding light on general principles of immune adaptation to chronic infections.
Moreover, these insights have profound implications for vaccine development, as they identify specific immune signatures associated with protective immunity versus susceptibility or pathology. Designing vaccines that elicit balanced Th1/Th2 responses, promote durable effector memory, and avoid premature exhaustion could revolutionize schistosomiasis control, a necessity given the limitations of current chemotherapy-based strategies.
The study also touches upon the potential impact of host genetics and environmental factors in shaping immune responses, although these were beyond the direct scope of the current work. Nevertheless, the standardized infection model paves the way for future investigations into personalized immunotherapies and the role of microbiome interactions in helminth infections.
In conclusion, this research not only elucidates the complex interplay between repeated schistosome infections and adaptive immunity but also exemplifies how cutting-edge immunological tools and controlled human models can accelerate understanding of neglected tropical diseases. By uncovering the detailed choreography of T cells in different exposure scenarios, the study opens new horizons for interventions that harness the immune system’s full potential to combat parasitic infections effectively and safely.
The findings are poised to inspire a paradigm shift in schistosomiasis research, encouraging the integration of controlled infection trials with molecular immunology to unravel host-pathogen interactions. As schistosomiasis remains a major global health burden, the insights gained here bring hope for innovative solutions that could tip the balance in favor of durable immunity and disease elimination.
Subject of Research: T cell immune responses to repeated controlled human schistosome infection versus natural exposure
Article Title: T cell responses in repeated controlled human schistosome infection compared to natural exposure
Article References:
Driciru, E., Koopman, J.P.R., Steenbergen, S. et al. T cell responses in repeated controlled human schistosome infection compared to natural exposure. Nat Commun 16, 6827 (2025). https://doi.org/10.1038/s41467-025-62144-8
Tags: adaptive immunity in parasitic infectionschronic schistosome infection effectscontrolled vs natural schistosome infectioneffector functions of T cellsflow cytometry in immune researchimmune responses to schistosome wormsimmunological memory in schistosomiasisschistosomiasis public health challengessingle-cell transcriptomics in immunologyT cell dynamics in schistosomiasisT cell phenotypes in schistosome exposurevaccine development for schistosomiasis