Credit: University of Tsukuba
Tsukuba, Japan – A little over 10 years ago, the first reprogramming of adult cells into undifferentiated stem cells was achieved. These induced pluripotent stem cells (iPSCs) have the ability to become almost any cell type and can divide indefinitely, so share many features with embryonic stem cells. Such characteristics enable iPSCs to be used in several applications of regenerative medicine, particularly because they can be derived from an individual's own cells so tissue rejection problems are not encountered. They can also be programmed to develop into rare or inaccessible cell types, used to screen novel drugs, and studied to understand the cellular basis of disease or reprogramming.
However, while the genetic factors responsible for reprogramming are well known, the mechanisms underlying the responses to induced gene expression changes are not as clear.
Now, research led by the University of Tsukuba has solved the mystery surrounding one of the reprogramming factors, KLF4. The study was published in Stem Cell Reports.
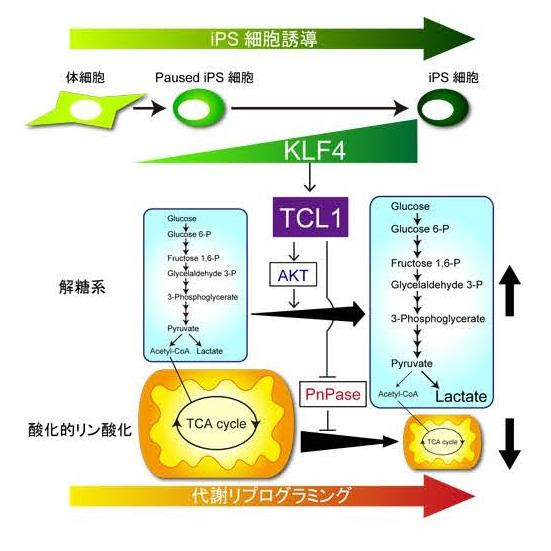
KLF4 together with other reprogramming transcription factors is used in the lab to force the expression of genes in somatic cells (adult non-germline cells) in the development of iPSCs. Somatic cells generate their energy in an oxygen-fueled process called oxidative phosphorylation, which takes place in the mitochondria, also known as cellular powerhouses.
In contrast, stem cells have small mitochondria and use glycolysis as an alternative biochemical pathway to generate energy. This series of reactions can be anaerobic, so more suited to their typically low-oxygen environment, but also provides the supply of metabolic intermediates necessary for rapid growth and division.
University of Tsukuba researchers developed a gene transfer system that allowed iPSC reprogramming to only occur in the presence of KLF4, thus focusing exclusively on its role in the process. They then used genome-wide analysis to search for genes switched on by KLF4 at a late stage of reprogramming.
"We found that the Tcl1 gene was upregulated by KLF4 binding to its enhancer and promoter regions," study co-first author Ken Nishimura says. "KLF4 also caused the binding of another reprogramming factor, OCT4, to the Tcl1 promoter."
The team discovered that the TCL1 protein played a key role in increasing glycolysis by activating a different metabolic pathway that is important for the self-renewal of stem cells.
"We also showed that TCL1 inhibits a mitochondrial enzyme required for in oxidative phosphorylation, leading to a reduction in oxygen consumption of the cells", co-first author Shiho Aizawa explains. "This was matched by increased glucose uptake for glycolysis, revealing that TCL1 promotes the metabolic switch in energy generation necessary for cells to acquire pluripotency."
###
The article, "A Role for KLF4 in Promoting the Metabolic Shift via TCLI during Induced Pluripotent Stem Cell Generation" was published in Stem Cell Reports at DOI: 10.1016/j.stemcr.2017.01.02
Media Contact
Masataka Watanabe
[email protected]
81-298-532-039
############
Story Source: Materials provided by Scienmag