In a groundbreaking new study, scientists have uncovered the pivotal role of Type I interferons in orchestrating the cytotoxic behavior of T cells through the upregulation of a key transcription factor, interferon regulatory factor 7 (IRF7), within the context of autoimmune kidney diseases in murine models. This discovery not only illuminates previously obscure mechanisms driving immune-mediated renal damage but also paves a promising pathway for the development of targeted immunotherapies aimed at halting progressive renal failure attributed to autoimmune assaults.
Autoimmune kidney diseases, characterized by immune system malfunction that precipitates tissue injury within the kidneys, present a significant clinical challenge due to their complex immunopathology and limited therapeutic options. It is well recognized that T lymphocytes, particularly cytotoxic subsets, contribute prominently to the inflammatory milieu and subsequent tissue destruction. However, the upstream molecular signals that enhance T cell cytotoxicity in the inflamed renal environment have remained elusive until now. The findings reported herein shed unprecedented light on how Type I interferon signaling induces IRF7, thereby amplifying the cytotoxic potential of T cells and exacerbating renal pathology.
Type I interferons, a family of cytokines best known for their antiviral responses, have increasingly been recognized for their immunomodulatory roles in autoimmunity and inflammation. While their systemic elevation is a hallmark of various autoimmune disorders, including systemic lupus erythematosus, the specific mechanisms by which Type I interferons influence effector T cell function in autoimmune nephritis had not been explicitly defined. The current study elucidates the molecular cascade initiated by Type I interferons that culminates in the transcriptional activation of IRF7, a master regulatory factor traditionally implicated in antiviral immunity but now revealed as a driver of T cell-mediated cytotoxic responses within the kidney.
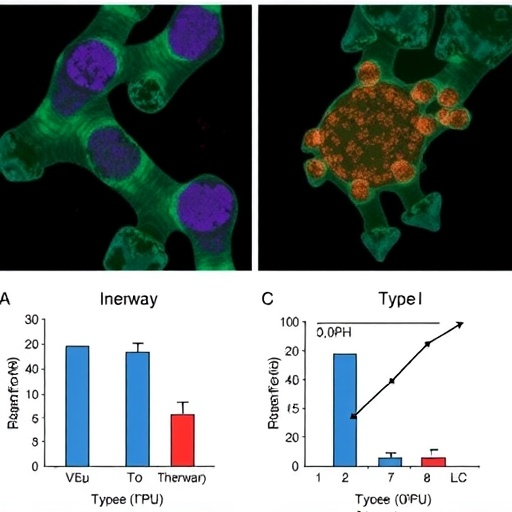
Delving into the cellular dynamics, the researchers employed sophisticated genetic and immunological tools to manipulate Type I interferon signaling pathways in murine models predisposed to autoimmune renal injury. By selectively abrogating interferon receptor expression or modulating IRF7 levels specifically in T cells, they demonstrated a direct correlation between Type I interferon signaling intensity, IRF7 expression, and the acquisition of a cytotoxic phenotype by CD8+ T lymphocytes infiltrating the kidney parenchyma. This cytotoxic phenotype was characterized by elevated expression of granzyme B, perforin, and other effector molecules deleterious to renal tissue integrity.
Notably, these insights were gleaned using advanced single-cell transcriptomics and flow cytometric analyses that allowed for high-resolution profiling of immune cell subsets within the inflamed kidneys. Through these cutting-edge techniques, the study identified discrete populations of IRF7-high T cells exhibiting enhanced cytotoxic functional signatures. These subpopulations provided a cellular foothold to unravel the interplay between Type I interferon signaling and T cell-mediated immunopathology, reinforcing the concept that IRF7 acts at a crossroads of innate and adaptive immune pathways within the kidney microenvironment.
Further validation of the pathogenic role of the Type I interferon–IRF7 axis was achieved through therapeutic intervention experiments. The researchers administered specific inhibitors targeting the interferon receptor or employed genetic knockdowns of IRF7, resulting in marked attenuation of T cell cytotoxicity and improvement of kidney function parameters in the autoimmune mice. This translational aspect underscores the potential of targeting this axis as a novel therapeutic avenue to ameliorate renal autoimmunity without broadly suppressing the immune system, which could carry significant infection risks.
An additional layer of complexity was revealed by observations that IRF7 modulation impacts not only cytotoxic T cells but also their crosstalk with other immune subsets, including dendritic cells and macrophages, which orchestrate inflammatory responses within the kidney. This intercellular communication appears to amplify local interferon production and sustain a chronic inflammatory circuit, highlighting the intricate cellular networking that perpetuates kidney damage. Interrupting this crosstalk by dampening IRF7 expression disrupted the inflammatory cascade and favored tissue repair mechanisms.
Moreover, the study’s insights have broad implications beyond autoimmune nephritis, given the conserved role of Type I interferons and IRF7 in various autoimmune diseases. The mechanistic elucidation provided here may translate to other organ-specific autoimmune pathologies where cytotoxic T cells contribute to tissue injury, such as autoimmune myocarditis or encephalitis. By defining IRF7 as a key molecular switch controlling cytotoxic T cell function under the influence of Type I interferons, this research opens fertile ground for cross-disease therapeutic strategies.
The integration of cutting-edge genomic, proteomic, and metabolic analyses presented in this work exemplifies the power of multidisciplinary approaches in immunology and nephrology. The precise dissection of molecular pathways enabled the authors to move beyond correlative observations to establish causative relationships underpinning disease progression. This integrative strategy sets a benchmark for future studies seeking to untangle complex autoimmune circuits and devise precision medicine interventions.
Furthermore, the temporal dynamics of Type I interferon signaling and IRF7 expression were explored, revealing that early phase induction of this pathway is necessary for initiating T cell cytotoxic programs, while sustained activation drives chronic inflammation and renal fibrosis. This temporal dimension emphasizes the importance of timely therapeutic interventions that could effectively interrupt disease progression before irreversible tissue scarring occurs, potentially preserving renal function in affected patients.
The implications of this research extend into the realm of biomarker discovery as well. Elevated IRF7 expression or its downstream cytotoxic mediators could serve as predictive markers of disease activity or treatment response in autoimmune kidney diseases. Leveraging these biomarkers might facilitate patient stratification, allowing clinicians to identify individuals most likely to benefit from therapies targeting the Type I interferon–IRF7 axis and monitor disease trajectories with greater precision.
Critically, while these findings are robust in murine models, translation to human autoimmune kidney diseases will require careful clinical validation. Nonetheless, the conservation of interferon pathways across species and the corroboration of elevated Type I interferon signatures in human lupus nephritis and other autoimmune nephropathies provide a strong rationale for advancing this line of research into clinical trials. Such trials could evaluate the efficacy and safety of IRF7-targeted therapies and potentially transform management paradigms.
As the scientific community digests these findings, the broader questions beckoning exploration include how environmental triggers modulate Type I interferon responses in the kidney, the interplay with other immune regulatory networks, and the potential compensatory mechanisms that might arise upon IRF7 inhibition. Future research tackling these challenges will undoubtedly refine our understanding and therapeutic arsenal against autoimmune kidney pathology.
In essence, this study not only advances fundamental knowledge of immune-mediated tissue injury but also exemplifies the transformative power of focused molecular investigations in unveiling new therapeutic targets. With autoimmune kidney diseases constituting a significant burden on global health, these insights arrive at a timely juncture, inspiring optimism for the development of groundbreaking treatments that could alter the trajectory of autoimmune nephritis.
Subject of Research: The role of Type I interferon in driving T cell cytotoxicity through upregulation of interferon regulatory factor 7 (IRF7) in autoimmune kidney diseases using murine models.
Article Title: Type I interferon drives T cell cytotoxicity by upregulation of interferon regulatory factor 7 in autoimmune kidney diseases in mice.
Article References:
Wang, H., Engesser, J., Khatri, R. et al. Type I interferon drives T cell cytotoxicity by upregulation of interferon regulatory factor 7 in autoimmune kidney diseases in mice. Nat Commun 16, 4686 (2025). https://doi.org/10.1038/s41467-025-59819-7
Image Credits: AI Generated
Tags: autoimmune kidney diseasescytokines in autoimmunityimmune-mediated tissue injuryimmunomodulatory roles of cytokinesimmunotherapy for renal failureinflammatory renal pathologyinterferon regulatory factor 7murine models of diseaseT cell cytotoxicityT lymphocyte activationtranscription factor regulationType I interferon