A groundbreaking advance in the realm of molecular chemistry has emerged from a dedicated team of scientists at The University of Hong Kong (HKU), in partnership with international researchers. Their latest work, published in the esteemed journal Nature Synthesis, unveils a compact catenane molecule exhibiting tunable mechanical chirality. This pioneering development holds immense potential to revolutionize fields such as materials science, nanotechnology, and pharmaceutical design by introducing a controllable form of chirality based not on traditional covalent bonding, but on the mechanical interlocking of molecular components.
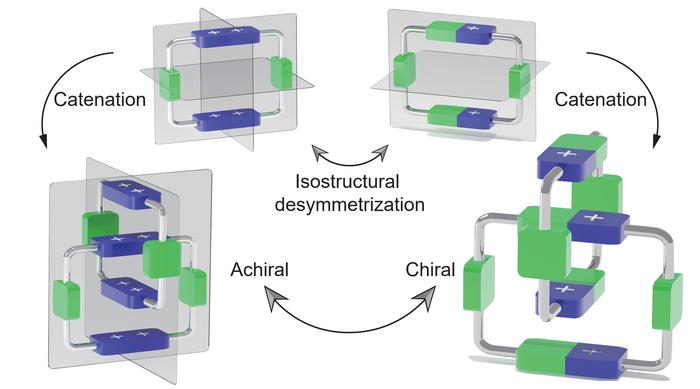
Catenanes represent a fascinating class of mechanically interlocked molecules comprising two or more macrocyclic rings intertwined akin to the links of a chain. Unlike conventional molecules, where atoms are connected through covalent bonds, catenanes are stabilized through their unique topological arrangement, granting them exceptional stability and distinct physical properties. The concept of mechanical chirality in these structures arises when the spatial configuration of interlocked rings lacks superimposability on their mirror images, imparting stereochemical uniqueness without relying on asymmetric atoms.
Delving into the chemistry, this research vividly demonstrates how two achiral molecular rings, each defined by specific symmetrical attributes, can be architecturally coaxed into forming a chiral catenane. This transformation is made possible through an innovative isostructural desymmetrisation strategy, which effectively disrupts the inherent symmetry without altering the molecular framework’s fundamental composition. The resultant catenane adopts a compact co-conformation that closely mirrors the shape of the achiral precursor but manifests new chiral characteristics due to the loss of individual ring symmetry once mechanically interlocked.
From a synthetic chemistry perspective, the team has devised an exquisite methodology that allows them to finely control the chirality of these catenanes. By introducing chiral disulfonate guest molecules, they are able to bias the equilibrium towards one enantiomeric form over its mirror image selectively. This dynamic chiral induction offers a powerful means to manipulate the molecule’s stereochemical outcome in both solution and crystalline states, paving the way for the design of responsive materials whose optical and mechanical properties can be externally modulated.
The structural compactness of these catenanes ensures a highly efficient interaction between the interlocked rings, which is crucial in maintaining their chiral conformation. Advanced computational modeling combined with experimental studies enabled the team to map the energy landscape of these mechanical bonds and to elucidate the mechanistic pathways enabling controlled interconversion between different chiral states. This synergy of theory and practice stands as a remarkable example of modern chemical research’s integrative approach to problem-solving.
One of the most fascinating aspects of this study lies in the tunability of mechanical chirality. By varying the molecular architecture and the presence of chiral guests, the researchers exert precise control over the switching behavior of the catenane’s chirality. This capability heralds the possibility of constructing molecular machines and devices that function based on mechanical stereochemistry, an area of enormous scientific intrigue and technological promise.
The implications of such tunable mechanostereochemistry extend deeply into nanotechnology, where molecular machines with predictable and controllable chiral functions could perform sophisticated tasks including molecular recognition, catalysis, and targeted drug delivery. The ability to reversibly switch chirality could allow these systems to respond to external stimuli or environmental changes, thereby enhancing their versatility and functional sophistication.
Moreover, in materials science, embedding such mechanically chiral catenanes into polymeric matrices or composite materials opens new horizons for generating materials with customized mechanical, optical, and electronic responses. Such materials could be tailored for advanced sensing platforms, stimuli-responsive coatings, or novel photonic devices, where chirality plays a fundamental role in modulating light-matter interactions.
This collaborative discovery was spearheaded by the late Nobel Laureate Professor Fraser Stoddart alongside Research Assistant Professors Chun Tang and Ruihua Zhang at HKU’s Department of Chemistry. Their work was complemented by experts from Northwestern University and ShanghaiTech University, reflecting an exemplary international synergy. The amalgamation of diverse expertise was vital to the project’s success, enhancing the molecular design, synthetic execution, and analytical characterization phases.
Beyond its scientific significance, this research pays tribute to the visionary leadership and scientific acumen of Professor Stoddart, whose profound contributions to supramolecular chemistry paved the groundwork for current innovations. His untimely passing in late 2024 was deeply felt across the research community, yet his legacy endures in this remarkable advancement embodying the spirit of molecular ingenuity.
Financial support from institutions such as the University Research Committee of HKU, the United States Department of Energy, and the Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study underpinned this research endeavor. These funding streams made possible the sophisticated experimental setups and computational resources essential for exploring the delicate interplay of mechanical bonding and chirality.
Looking forward, the development of compact catenanes with tunable mechanical chirality promises to fuel future discoveries in chemical synthesis and molecular engineering. The capacity to design molecules wherein chirality is governed mechanically rather than covalently presents a transformative paradigm in stereochemistry that can influence drug development, enantioselective catalysis, and the fabrication of dynamic materials.
In summary, the intricate manipulation of mechanical chirality within catenane architectures not only broadens our fundamental understanding of stereochemistry but also drives forward the frontiers of material innovation and molecular machinery. The blend of chemical creativity, precise synthetic control, and computational insight showcased in this work underscores the exciting scientific possibilities residing at the interface of mechanics and molecular design.
Subject of Research: Not applicable
Article Title: A compact catenane with tuneable mechanical chirality
News Publication Date: 14-Apr-2025
Web References: http://dx.doi.org/10.1038/s44160-025-00781-z
Image Credits: The University of Hong Kong
Keywords
Physical sciences, Applied sciences and engineering
Tags: achiral to chiral transformationcompact catenane molecule developmentHKU chemists catenane mechanical chiralityinterlocked molecular structuresmaterials science applicationsmolecular chemistry advancesnanotechnology innovationsNature Synthesis publicationpharmaceutical design implicationsstereochemical uniqueness in catenanestopological arrangement of moleculestunable mechanical chirality