The intricate interplay between RNA-binding proteins (RBPs) and cancer biology has garnered significant attention in recent years, with the fragile X mental retardation protein (FMRP) emerging as a particularly compelling figure. Historically known for its pivotal role in neural development and synaptic function, FMRP’s involvement in oncology challenges prevailing assumptions and opens novel vistas for therapeutic intervention. A comprehensive review recently published in Genes & Diseases meticulously details the multifaceted roles of FMRP, emphasizing its dualistic functions as both tumor suppressor and promoter, thereby deepening our understanding of RNA metabolism’s complex influence on oncogenesis.
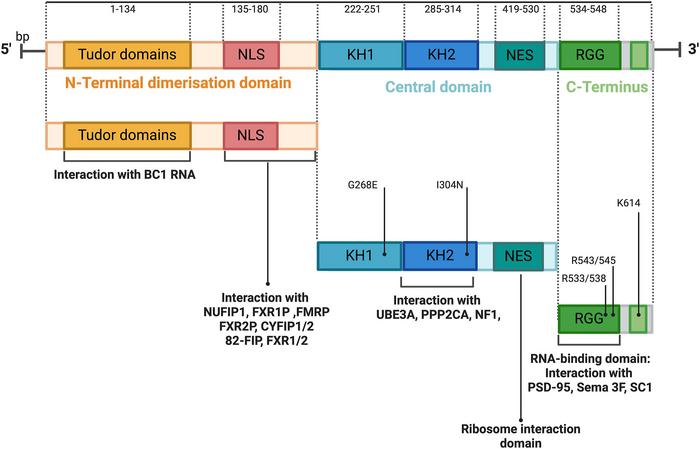
FMRP is a multidomain RNA-binding protein characterized by its tandem Tudor domains at the N-terminus, crucial for recognizing methylated lysine residues on partner proteins. This interaction is instrumental in regulating epigenetic landscapes and influencing RNA processing pathways, which together shape gene expression profiles requisite for normal cellular homeostasis and pathogenesis. Through modulating mRNA stability, transport, and translation, FMRP positions itself as an indispensable regulator within the post-transcriptional control mechanisms that orchestrate tumor progression, cell proliferation, and cellular plasticity.
The dichotomy of FMRP’s function in cancer is underscored by its context-dependent roles. In certain cellular milieus, diminished FMRP expression correlates with a reduction in tumorigenic potential, suggesting that its presence may support oncogenic processes. Conversely, elevated levels of FMRP have been documented in aggressive malignancies, notably breast, colorectal, and hepatocellular carcinomas. These observations point toward a complex regulatory network where FMRP either constrains or facilitates oncogenesis, possibly linked to heterogeneous tumor microenvironments and genetic backgrounds that dictate cellular responses to this RNA-binding protein.
One striking aspect delineated in the review is FMRP’s involvement in therapy resistance—a formidable challenge that undermines cancer treatment efficacy. By stabilizing oncogenic mRNAs such as epidermal growth factor receptor (EGFR) transcripts in colorectal cancers, FMRP potentiates proliferative signaling pathways, ultimately fostering tumor growth and survival under therapeutic assault. This post-transcriptional regulation mechanism not only enables cancer cells to evade apoptosis induced by chemotherapy or radiation but also modulates key pathways implicated in immune evasion, highlighting FMRP as a central node in tumor resilience.
In addition to its influence on growth and survival signaling, FMRP significantly contributes to tumor metastasis through pathways such as epithelial-mesenchymal transition (EMT). EMT is a critical process whereby epithelial cells acquire mesenchymal phenotypes, enhancing migratory and invasive capabilities. The protein’s regulation of mRNAs encoding EMT-associated factors underscores its role in orchestrating cellular plasticity, a hallmark of metastatic dissemination. As metastasis remains the leading cause of cancer-related mortality, deciphering how FMRP mediates EMT provides valuable insights for intervention strategies aimed at curbing cancer spread.
The intersection of FMRP function and immunology is another frontier explored within the review. Emerging evidence implicates FMRP in modulating interactions within the tumor microenvironment, particularly regarding immune surveillance and checkpoint inhibition. Its influence on mRNA stability and translation of immune regulators may contribute to an immunosuppressive milieu, assisting tumor cells in evading immune-mediated destruction. This facet positions FMRP not merely as a regulator of cancer cell intrinsic pathways but also as a mediator of extrinsic interactions that impact therapeutic outcomes, especially in the context of immunotherapy.
Given these diverse roles, targeting FMRP therapeutically presents both challenges and opportunities. Direct inhibition may impair essential physiological functions, especially in the nervous system, yet selective modulation of its oncogenic activities could yield substantial clinical benefits. The review highlights the promise of precision medicine approaches, wherein understanding the post-translational modifications (PTMs) and protein-protein interactions that govern FMRP’s activity may enable the design of highly specific interventions. Such strategies might selectively curb tumor-promoting functions of FMRP while preserving or even enhancing its tumor-suppressive roles.
Unraveling the PTMs of FMRP, including phosphorylation, methylation, and ubiquitination, is pivotal to this endeavor. These chemical modifications alter the protein’s conformation, binding affinity, and interaction networks within the cell, thereby fine-tuning its regulatory repertoire. Insights into the dynamic regulation of FMRP will inform drug development pipelines aimed at modulating its activity in precise temporal and spatial manners, circumventing the off-target effects that have historically limited RNA-binding protein therapeutics.
The review further stresses the importance of considering FMRP within the broader RBP landscape. RNA-binding proteins constitute an extensive network that collectively orchestrates RNA fate decisions, affecting transcriptome stability and translation. Aberrations in this network are increasingly recognized as drivers of tumorigenesis. Integrative analyses that contextualize FMRP’s functions alongside other RBPs could elucidate coordinated regulatory circuits amenable to combinatorial therapeutic targeting, which may provide enhanced efficacy over single-target approaches.
Moreover, the utility of FMRP as a biomarker for cancer diagnosis and prognosis is gaining traction. Its expression levels, subcellular localization, and interaction profiles could inform disease states and predict therapeutic responsiveness. Incorporating FMRP quantification into clinical workflows may facilitate stratification of patients who are likely to benefit from specific treatments, thereby personalizing care and improving clinical outcomes.
In sum, the evolving narrative of FMRP in cancer biology exemplifies the complexity of RNA-based regulation in disease. It challenges previous paradigms that relegated RBPs merely as supportive factors, positioning them instead as active and versatile agents in tumorigenesis. Continuous research is essential to decode the nuanced roles of FMRP, particularly its paradoxical dualism, which embodies both the promise and challenge of targeting multifaceted molecular players in cancer.
This groundbreaking synthesis of molecular biology and oncology enriches our understanding and propels forward a new frontier where RNA-binding proteins like FMRP could transform cancer diagnosis and therapy. The integration of mechanistic insights with clinical translation holds the key to unlocking precision medicine strategies that effectively harness the dualistic nature of FMRP—turning a protein once primarily linked to neural disorders into a pivotal target in the fight against cancer.
Subject of Research: The multifaceted role of RNA-binding protein FMRP in cancer progression and therapy resistance.
Article Title: The role of RNA binding proteins in cancer biology: A focus on FMRP.
News Publication Date: Not specified.
Web References: DOI link – http://dx.doi.org/10.1016/j.gendis.2024.101493
References: Yunlu Jia, Ruyin Jia, Yongxia Chen, Xuanyi Lin, Nadire Aishan, Han Li, Linbo Wang, Xiaochen Zhang, Jian Ruan, Genes & Diseases, 2025, 101493.
Image Credits: Genes & Diseases
Keywords: Carcinogenesis, RNA-binding proteins, FMRP, tumor progression, therapy resistance, metastasis, epithelial-mesenchymal transition, precision medicine, post-translational modifications, RNA metabolism, immune evasion.
Tags: cellular plasticity in tumorsdual role of FMRP in tumorsepigenetic regulation in cancerFMRP and cancer biologyfragile X mental retardation protein functionsmRNA stability and cancerpost-transcriptional control mechanismsRNA metabolism in cancerRNA-binding proteins and oncogenesistherapeutic interventions targeting FMRPtumor progression and therapy resistancetumor suppressor and promoter roles of FMRP