Fukuoka, Japan—Deep within the intricate tapestry of human life, numerous cells persistently navigate through the vascular network of our bodies. From the ubiquitous red blood cells facilitating oxygen transport, to the dedicated immune cells diligently safeguarding against pathogens, the ceaseless movement of these microscopic entities is a marvel of biological engineering. Proven to be a critical area of study, the fluid dynamics governing these cells hold the potential to reshape medical devices and enhance artificial organs, particularly artificial hearts. Yet, despite the extensive implications of this research, capturing and analyzing the behaviors of these flowing cells in real time has proven exceedingly difficult.
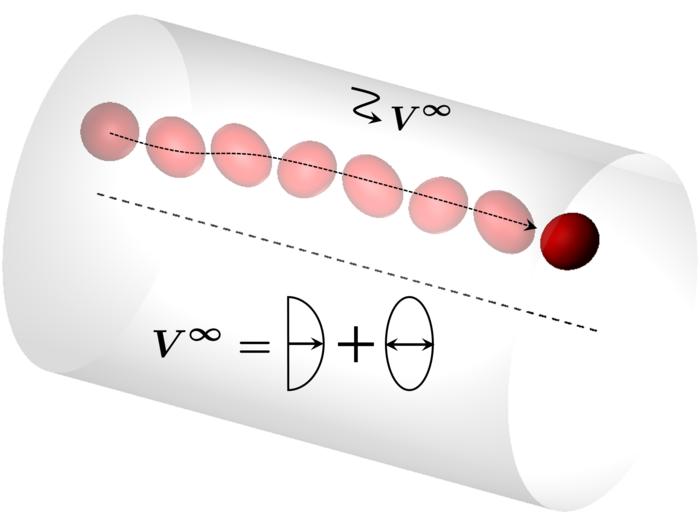
In a significant advancement, researchers from Japan recently unveiled their innovative approach towards simulating the complex fluid dynamics of flowing biological cells. Publishing their findings in the reputable Journal of Fluid Mechanics, they utilized a novel technique involving numerical simulations to craft an in-silico cell model. This model conceptualizes biological cells as deformable capsules operating within a pulsating flow, ideally replicating how these cells traverse through the blood vessels. The intricate study hinges on understanding the specific positions that the capsules occupy, regulated largely by their deformation and the frequency of the pulsating flow.
A notable breakthrough demonstrated through these simulations is the discovery of a pulsation frequency that induces a remarkable oscillation in the capsules, compelling them to migrate away from the tube’s center, a region characterized by rapid flow, towards zones where the flow is comparatively slower. This insight provides researchers with a vital tool to discern not just the mechanics of cell movement within the vascular system, but also the implications of pulsating flow behavior under varying conditions.
The behavior of biological cells, especially when subjected to the complexities of unsteady flow, is an intricate dance reflected in their fluid dynamics. As these cells navigate through an array of blood vessels, differing in size and composition, their inherent flexibility permits them to stretch and deform, affecting their movement patterns. This understanding of deformation is integral to the research, revealing that flow speed can interact with pulsation frequencies to influence how well cells can navigate their environment.
In an explanation by Associate Professor Naoki Takeishi of Kyushu University, who spearheaded the research endeavor, he articulates the innovative nature of their numerical modeling approach. This sophisticated model allows them to step beyond conventional boundaries of fluid dynamics, effectively simulating the multifaceted movement of cells in pulsating tubes. The utilization of extensive computational resources confirms the commitment of the team to reveal the complexities of their findings with unprecedented accuracy.
The numerical simulations conducted by the research team led to an intriguing revelation regarding the effect of flow conditions on capsule behavior. Under conditions of fast flow, a consistent pulsation frequency is observed that enables stable movement of the capsules toward slower flow regions, despite fluctuations in flow speed. Conversely, under slower flow conditions, the capsules exhibited a proclivity to converge quickly to the center of the tube. These findings imply that understanding the relationship between deformation, pulsation, and flow speed could have profound implications for medical physiology.
Crucially, the developed model could serve pivotal roles in research aimed at the precise manipulation of cells and fluids, leading to practical applications such as cell sorting, aligning, and their ultimate isolation for therapeutic purposes. Such techniques are particularly valuable in oncology for capturing circulating tumor cells from patients, potentially paving the way for better diagnostic and therapeutic interventions.
Furthermore, the implications of unsteady versus steady blood flow in artificial organs, such as hearts, remain an area of contention among scientists and engineers alike. With no definitive consensus on which flow type is ideal for optimal performance, the foundational insights derived from this study could significantly influence the future design and functionality of artificial hearts and vascular components.
As the researchers further refine their simulations and explore the implications of their findings, the prospect of merging computational fluid dynamics with biological cell behavior emerges as a powerful paradigm shift. Ultimately, the knowledge gleaned from their research not only propels forward our understanding of basic physiological mechanics but also opens new avenues in creating advanced, life-saving medical devices tailored for improved human health.
By examining the intricate interactions at play within the fluid dynamics of biological entities, researchers at Kyushu University embody the spirit of innovation aimed at addressing some of society’s most pressing healthcare challenges. As we advance into an era fueled by technology and health science convergence, their work stands as a testament to the potential embedded within computational modeling as a transformative tool for the future of medical research and application.
In conclusion, the research team at Kyushu University represents a beacon of hope, challenging existing paradigms and harnessing the power of numerical modeling to unveil hidden dynamics of cellular movement. By providing the tools necessary to manipulate and understand cellular behavior within the dynamic ecosystem of our bodies, they not only enrich scientific knowledge but also enhance the potential for breakthroughs in the design of life-sustaining medical technologies.
Subject of Research: Fluid dynamics of biological cells
Article Title: Inertial focusing of spherical capsule in pulsatile channel flows
News Publication Date: 9-Apr-2025
Web References: Kyushu University, Journal of Fluid Mechanics
References: Journal of Fluid Mechanics
Image Credits: Takeishi Lab/Kyushu University
Keywords: fluid dynamics, biological cells, numerical simulation, pulsating flow, artificial organs, cancer research, computational modeling, cardiovascular health, medical technology
Tags: advancements in biological engineeringartificial organs and medical devicesdeformable capsules in blood flowfluid dynamics of biological cellsimmune cells and pathogen defenseJournal of Fluid Mechanics researchmapping cell locations in human bodynumerical simulations in cell modelingpulsating flow in vascular networksreal-time analysis of cell movementred blood cells and oxygen transportsimulation techniques in biomedical studies