The endoplasmic reticulum (ER) is a crucial cellular organelle responsible for protein synthesis, folding, and quality control. This system ensures that proteins attain their required three-dimensional conformations necessary for effective cellular functionality. Misfolded or improperly synthesized proteins can lead to a state of ER stress, triggering various cellular responses, including the activation of the unfolded protein response (UPR). Initially, the UPR acts as an adaptive mechanism, attempting to restore cellular homeostasis. However, prolonged ER stress can result in maladaptive responses, leading to cell death, which raises significant concerns regarding its implications in metabolic diseases.
Recent research led by an esteemed team from the Institute of Biophysics at the Chinese Academy of Sciences, spearheaded by Professor WANG Likun, unveils a groundbreaking intercellular signaling mechanism linking ER-stressed adipocytes to hepatocytes. This novel research emphasizes how adipocytes under pathological conditions manage to influence liver cell function through a signaling molecule known as ceramide. Ceramide, a bioactive sphingolipid, plays an instrumental role in cellular signaling pathways, particularly regarding stress responses, cell survival, and death. The findings from this study significantly contribute to our understanding of metabolic dysregulation and open new avenues for potential therapeutic interventions.
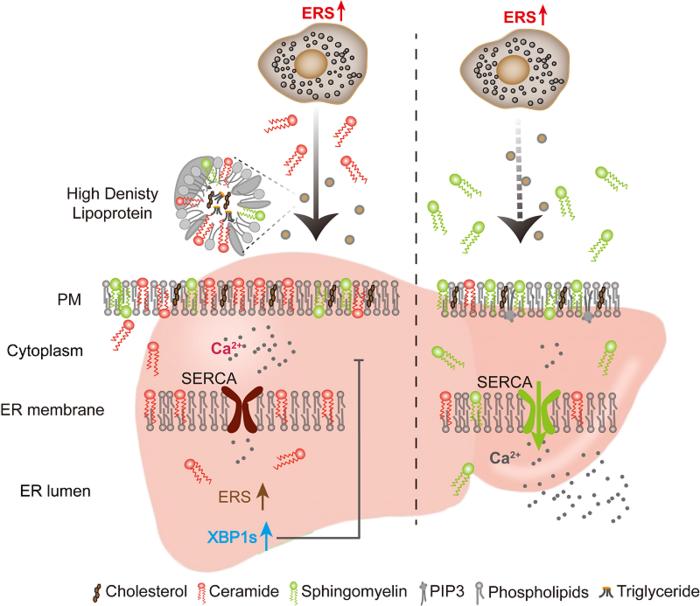
The study elucidated that ceramide secretion occurred as a response to ER stress in adipocytes, demonstrating its capability to activate the UPR in nearby hepatocytes. This critical discovery highlights a sophisticated method of intercellular communication, wherein adipose tissue can exert systemic effects on liver function, revealing a previously unappreciated level of organ crosstalk in human physiology. Through rigorous experimental analysis, the researchers confirmed that ER-stressed adipocytes release ceramide in quantities sufficient to instigate UPR signaling in liver cells across various tissues.
Utilizing lipidomics—a comprehensive analysis of lipid species—researchers identified that the stress-induced release of ceramide was not directly driven by the UPR mechanisms intrinsic to adipocytes. Rather, ER stress prompted the extracellular hydrolysis of sphingomyelin into ceramide, mediated by acid sphingomyelinase (ASM). This mechanism delineates an indirect regulatory pathway by which adipocytes communicate their state of health or distress to hepatocytes, suggesting a complex interaction between different cell types within the organism.
Transporting ceramide between adipocytes and hepatocytes involves high-density lipoprotein (HDL), which acts as a pivotal carrier of this signaling molecule. Upon arriving at the liver, ceramide impacts membrane fluidity, subsequently influencing the functionality of membrane-bound proteins. This alteration enhances the UPR’s activation state within hepatocytes, which is crucial for the cell’s ability to manage stress. Furthermore, the study suggests that modulating ceramide levels might delineate new therapeutic strategies for mitigating metabolic disorders tied to ER stress.
An interesting aspect of this research showed that reversing ceramide-induced membrane fluidity alterations was plausible through the supplementation of sphingomyelin. This finding highlights the possibility of restoring balance within cellular lipid metabolism, demonstrating that manipulating ceramide levels can have profound impacts on cellular function. It raises the prospect that maladaptive responses to ER stress might not be permanent and could indeed be reversible through strategic intervention involving lipid metabolism.
The implications of these findings extend beyond merely understanding lipid signaling pathways. They provide a broader framework for examining how various tissues coordinate their responses to stress, especially in the context of metabolic diseases. The research illuminates the notion that disruptions in lipid metabolism can have cascading effects throughout the organism, significantly influencing health outcomes. It also emphasizes the importance of maintaining lipid homeostasis as a critical factor in preventing the onset of metabolic disorders.
Understanding the mechanisms of intercellular signaling through ceramide sheds light on potential interventions targeting lipid metabolism. By aiming at the pathways involved in ceramide synthesis and transport, new therapeutic strategies could be devised to alleviate the symptoms of metabolic diseases that arise from chronic ER stress. Such an approach could fundamentally change how metabolic disorders are treated, focusing not on the symptoms but rather on the underlying cellular communication failures that lead to these conditions.
Moreover, the insights gained from this research may lay the groundwork for further investigations into how similar signaling mechanisms operate across various tissues. With metabolic diseases on the rise globally, identifying ways to intervene in lipid signaling pathways could hold the key to developing effective treatments for an array of conditions characterized by metabolism-related stress responses.
In conclusion, the discoveries made by Professor WANG Likun and his team significantly advance our understanding of cellular communication in the context of ER stress and metabolic disease. By elucidating the role of ceramide in mediating intercellular signals between adipocytes and hepatocytes, this research contributes a vital piece to the puzzle of metabolic health. As scientists continue to explore these relationships, there lies great potential in unraveling new therapeutic avenues that will promote health and well-being in the face of increasingly prevalent metabolic disorders.
Subject of Research:
Article Title:
News Publication Date:
Web References:
References:
Image Credits:
Keywords
Tags: adipocyte pathology and liver functionadipocyte-hepatocyte communicationbioactive sphingolipids in cellular signalingcellular stress and survival mechanismsceramide signaling pathwaysendoplasmic reticulum stress responseimplications of ER stress in health.intercellular signaling in metabolismmetabolic disease implicationsprotein synthesis and folding in cellstherapeutic interventions for metabolic dysregulationunfolded protein response mechanisms