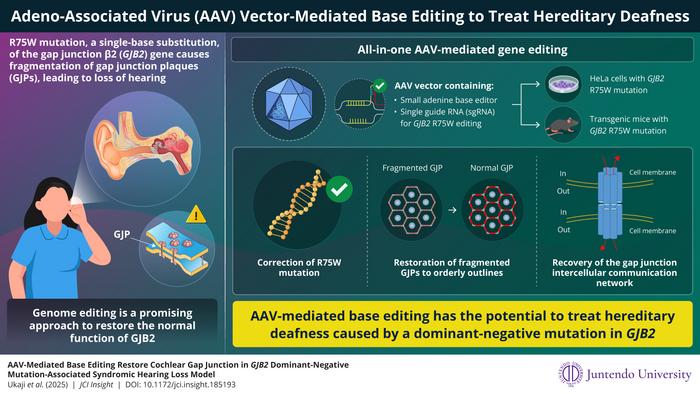
In groundbreaking advances in the field of genetic medicine, researchers from Japan have pioneered a revolutionary gene therapy targeting syndromic hearing loss caused by a mutation in the GJB2 gene. This innovative approach seeks to address the pressing need for curative therapies that are currently absent for a significant proportion of patients suffering from genetic hearing loss. By utilizing an advanced gene-editing technology, researchers aim to repair the R75W mutation—a dominant-negative variant of the GJB2 gene responsible for a substantial number of hereditary deafness cases. The work, led by Associate Professor Dr. Kazusaku Kamiya and his skilled team at Juntendo University, is set to change the landscape of genetic therapies for auditory disorders.
Congenital hearing loss presents a significant challenge, affecting millions worldwide. Estimates suggest that genetic factors are responsible for approximately half of all instances of hereditary hearing loss, with the GJB2 gene being a pivotal player. Encoding the protein Connexin 26 (CX26), GJB2 forms crucial intercellular gap junctions that facilitate the exchange of ions and signaling molecules between cells, particularly in the delicate structure of the cochlea. The disturbance in the function of these gap junctions due to mutations can lead to severe auditory impairments.
The implications of GJB2 mutations are profound and complex. Typically, recessive mutations can be addressed through gene replacement therapies. However, dominant-negative mutations, like R75W, pose a more formidable challenge as they not only disrupt the function of the mutated gene but also inhibit the normal function of the wild-type gene. This characteristic necessitates the development of precise gene editing capabilities to effectively restore auditory function and structural integrity.
With this background, the research team from Juntendo University embarked on a mission to create a viable gene therapy solution for individuals carrying the R75W mutation. Their preliminary findings highlighted the lack of existing therapeutic interventions for this condition, emphasizing the urgent need for innovative approaches to tackle hereditary hearing loss. In their quest, the researchers developed a tailored adeno-associated virus vector (AAV-Sia6e), which possesses an enhanced capacity for targeting and entering the specialized inner ear cells, known as hair cells, pivotal for hearing.
One pivotal aspect of their research was the miniaturization of the base editing tool, which encompassed the dimerization of SaCas9-NNG and ABE8e (adenine base editor). This innovative approach circumvents the size limitations inherent to conventional gene delivery systems. By streamlining the gene-editing tool, the team ensured its compatibility with the AAV vector they engineered. This seamless integration not only broadened the therapeutic application but also optimized the efficiency of the gene-editing process.
Their comprehensive methodology seamlessly led to the successful uptaking and execution of precise genetic modifications in cultured human cells exhibiting the GJB2 R75W mutation. The on-target editing resulted in a specific T-to-C conversion that restored normal gap junction formation, thereby reinstating the physiological functionality of intercellular communication—a critical component in maintaining auditory health. This remarkable outcome underscores the utility of AAV-mediated genome editing as a viable route for addressing genetic forms of hearing impairments.
To validate their hypothesis further, the research team took their studies to the next level by employing a transgenic mouse model specifically developed to exhibit the GJB2 R75W mutation. Through the evaluation of cochlear samples post-AAV-mediated editing, significant improvements were noted. The distinct formation of gap junctions in the transgenic mice mimicked the structures found in wild-type specimens, illustrating the precision and efficacy of their editing strategy.
Dr. Kamiya elucidated the broader implications of their research, indicating that their all-in-one AAV vector could pave the way for therapeutic advancements not only in hearing loss associated with GJB2 but also in other genetic disorders involving gap junctions. The potential to develop cost-effective and broadly applicable gene therapies signals a transformative era in treating genetic hearing loss, offering hope to countless individuals yearning for effective interventions.
Moreover, the researchers highlighted the anticipated safety advantages of their approach over existing CRISPR-Cas9 gene-editing technologies. The ABE-based method is projected to yield lower cytotoxicity rates, contributing to a safer treatment paradigm for patients. With safety and efficiency prioritized, the implications of this research extend beyond addressing hereditary hearing loss; they open avenues for exploring genetic therapies across a wider spectrum of conditions.
Through significant advancements in gene-editing technologies, these findings stand to reshape the therapeutic landscape for hereditary deafness. As the incidence of genetic hearing loss rises, the critical nature of such research becomes even more apparent. The potential for future adaptations of their AAV-mediated base editing technology to target other mutations causing auditory impairments reinforces the urgency and relevance of this work.
In summary, the dedication and innovative spirit exhibited by Dr. Kamiya and his research team reflect a commitment to advancing the frontiers of genetic therapy. Their research elucidates a pathway towards effective interventions that aim to restore hearing and improve the quality of life for thousands afflicted by genetic auditory disorders. The promise of AAV-mediated genome editing resonates far beyond the realm of hearing loss, heralding a future where genetic diseases may be met with effective and accessible remedies.
This groundbreaking research was published in the esteemed journal JCI Insight, underscoring its significance and potential impact within the scientific community. As the field of genetic medicine continues to push the boundaries of possibility, advancements like those presented by Dr. Kamiya’s team illuminate the future of healing through science.
Subject of Research: Animals
Article Title: AAV-mediated base editing restores cochlear gap junction in GJB2 dominant-negative mutation-associated syndromic hearing loss model
News Publication Date: 10-Mar-2025
Web References: https://doi.org/10.1172/jci.insight.185193
References:
Image Credits: Credit: Dr. Kazusaku Kamiya from Juntendo University, Japan
Keywords
Tags: advanced gene-editing technologyauditory disorders genetic therapiesConnexin 26 and hearingcurative therapies for hearing impairmentDr. Kazusaku Kamiya researchgene therapy for hearing lossgenome editing for genetic deafnessGJB2 gene mutation treatmenthereditary deafness genetic factorsinnovative approaches to congenital hearing lossJuntendo University genetic researchR75W mutation in GJB2