Ferroptosis, a form of iron-dependent regulated cell death, is emerging as a critical player in various biological processes, particularly in the context of immune regulation and suppression. Unlike traditional apoptotic pathways, ferroptosis is characterized by the accumulation of lipid peroxides and iron-dependent reactive oxygen species (ROS). This unique mechanism not only exposes a new dimension of cell death but also brings forth potential implications in the treatment of diseases where the immune system plays a decisive role, such as cancer and autoimmune disorders.
At the heart of ferroptosis lies the cystine/glutamate antiporter system xc−, which is responsible for regulating the levels of antioxidant glutathione (GSH). When this system is disrupted, a toxic accumulation of lipid peroxides occurs, setting the stage for ferroptosis. This cellular event is juxtaposed with other forms of cell death, where immunity and immunogenicity differ significantly. Such distinctions highlight ferroptosis as more than a mere death pathway; it signifies a pivotal influence on the immune environment.
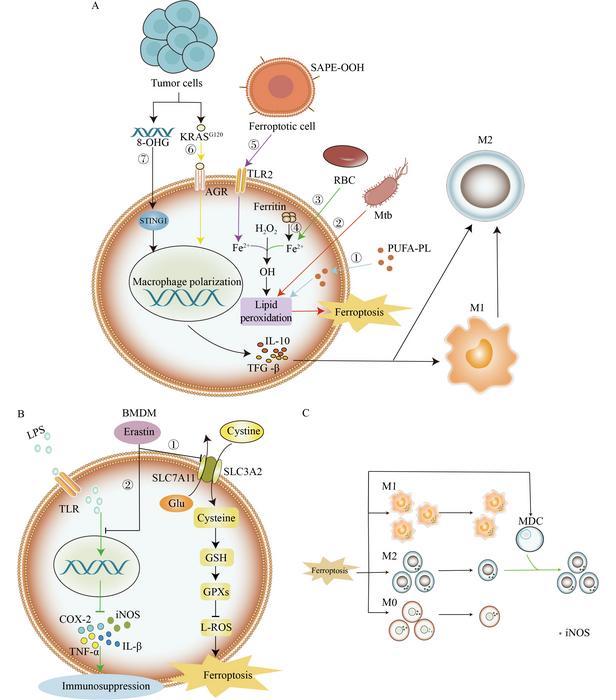
The role of macrophages in immune response is well-documented, yet their fate under ferroptotic conditions reveals a complex balance of immunity and suppression. Agents like erastin and RSL3, known to induce ferroptosis, have been shown to diminish the secretion of pro-inflammatory cytokines from macrophages. Under tumor microenvironments, factors such as 8-hydroxyguanosine (8-OHG) further push macrophages toward an M2 phenotype, which is associated with immunosuppression. This transformation underscores the susceptibility of M1 macrophages, which possess higher ferritin levels, to ferroptosis compared to their M2 counterparts. Inflammatory conditions coupled with pathogen interactions trigger macrophage ferroptosis, resulting in the release of harmful lipid peroxides and iron, thereby leading to systemic immunosuppression.
Neutrophils, as frontline defenders against pathogens, experience a similar fate. Our understanding of ferroptosis suggests that neutrophils, especially in the context of stroke and systemic lupus erythematosus (SLE), succumb to cell death due to diminished expression of PPAR-γ and GPX4. These changes directly impair their immune functions. Interestingly, with aging, neutrophils display an elevated sensitivity to ferroptosis, which may influence the progression of neurodegenerative diseases such as Alzheimer’s disease, highlighting the interplay between cellular aging and immune efficacy.
T cells, which comprise distinct subsets like Th1, Th2, and T regulatory cells (Tregs), are also influenced by ferroptosis. Within the tumor microenvironment, tumor cells exploit ferroptosis to undermine T cell function, utilizing mechanisms like CD36 expression and lipid ROS accumulation. This dynamic not only diminishes anti-tumor immunity but also leads to the survival of Tregs, which exhibit heightened resistance to ferroptosis and consequently suppress immune responses more effectively. Among T cell subsets, follicular helper T cells are particularly vulnerable to ferroptosis, suggesting a differential impact on T cell functionality which could have far-reaching implications in cancer therapy.
Moreover, B cells, the key players in humoral immunity, also show a distinct response to ferroptosis. In conditions like lupus nephritis, ferroptosis in renal epithelial cells can release immunosuppressive factors that negatively affect B cell functionality. The diversity among B cell subsets exists not only in their roles but also in their susceptibility to ferroptosis, with B1a cells demonstrating a heightened sensitivity compared to others.
Natural killer (NK) cells, essential for recognizing and eliminating tumor cells, are another target of ferroptotic processes in tumor microenvironments. Through mechanisms such as PD-L1 binding and the release of prostaglandin E2 (PGE2), tumor cells can induce ferroptosis in NK cells, thus attenuating their ability to mount a robust immune response. This phenomenon further complicates our understanding of the tumor-immune interface, underscoring the strategic adaptations that tumors utilize to evade immune detection.
Dendritic cells (DCs), fundamental for T cell activation, are not immune to the effects of ferroptosis. In various contexts, particularly within tumor environments, DCs undergoing ferroptosis lose their antigen-presenting capability. This loss critically hampers the initiation of T cell responses, with implications in both cancer progression and other pathologies such as sepsis and atherosclerosis. The interplay between ferroptosis and dendritic cell functionality may provide insights into developing targeted therapies for enhancing anti-tumor immunity.
Myeloid-derived suppressor cells (MDSCs), which are notorious for inhibiting immune responses, display subtypes with varying resistance levels to ferroptosis. Tumor-associated MDSCs exhibit remarkable resilience against ferroptosis, allowing them to thrive in immunosuppressive environments. Their adaptation enables them to outcompete other immune cell types for cystine, further depriving these cells and fostering tumor immune evasion.
Understanding the overarching impact of ferroptosis reveals its dual role in shaping immune responses. While various studies hint at possible immune-enhancing effects under certain circumstances, the consensus leans towards an overall immunosuppressive influence, particularly in chronic conditions and cancer. The resistance exhibited by some immune cell types, alongside their interactions with the microenvironment, presents fertile ground for novel therapeutic strategies aimed at repurposing ferroptosis to bolster immune reactions in patients with malignancies or infections.
As research continues, unraveling the intricate mechanisms governing ferroptosis in immune cells will be paramount. Insight into how these processes can be manipulated holds promise for developing effective treatments that can counteract the immunosuppressive effects of ferroptosis in diseases such as cancer, autoimmune disorders, and chronic inflammatory diseases. Future investigative efforts should prioritize a deep dive into the cellular and molecular pathways involved in ferroptosis, aiming to translate mechanistic understanding into clinical applications that enhance immune system efficacy.
By advancing knowledge in this area, the scientific community can aspire to identify new therapeutic targets and strategies that leverage the principles of ferroptosis, transforming the landscape of treatment for various diseases and paving the way toward improved patient outcomes.
Subject of Research: Not applicable
Article Title: Ferroptosis contributes to immunosuppression
News Publication Date: 24-Feb-2025
Web References: http://dx.doi.org/10.1007/s11684-024-1080-8
References: Not available
Image Credits: Nina He, Dun Yuan, Minjie Luo, Qing Xu, Zhongchi Wen, Ziqin Wang, Jie Zhao, Ying Liu
Keywords: Ferroptosis, immune suppression, macrophages, neutrophils, T cells, B cells, NK cells, dendritic cells, MDSCs, cancer, immune response.
Tags: cystine/glutamate antiporter system xc−ferroptosis and immune regulationferroptosis in autoimmune disordersglutathione levels and ferroptosisimmunogenicity and ferroptosisimplications of ferroptosis in cancer treatmentiron-dependent cell death mechanismslipid peroxides and immune suppressionmacrophages and immune responsepro-inflammatory cytokines and ferroptosisreactive oxygen species in immune regulationunique mechanisms of cell death