Lithium-6, a critical isotope for the advancement of nuclear fusion technology, has long posed significant challenges in terms of isolation and purification. Traditionally, this cumbersome process involved the use of liquid mercury—a highly toxic substance—under the COLEX method, which has been prohibited in the United States since 1963 due to environmental and health concerns. This limitation has forced scientists to depend on dwindling stockpiles, primarily maintained at Oak Ridge National Laboratory, for their experimental needs related to lithium-6. However, exciting new developments from researchers at ETH Zürich and Texas A&M University introduce a revolutionary mercury-free technique, generating hope for sustainable nuclear energy.
Sarbajit Banerjee, the senior author and a leading chemist, explained that the team’s innovation is a significant stride towards eliminating a longstanding impasse in the pursuit of nuclear energy and making lithium-6 more accessible for various applications. He notes that this methodological advancement could be a game-changer for the future of nuclear fusion, a clean energy source long sought by scientists and energy policymakers alike. As the world races for sustainable energy solutions, the implications of improved access to lithium-6 could resonate far beyond nuclear applications.
The innovative technique that allows for lithium-6 enrichment emerged serendipitously while the team was exploring methodologies for purifying “produced water,” which refers to the contaminated groundwater that surfaces during oil and gas extraction. During their investigations, researchers discovered that their newly developed membranes were exceptionally selective at capturing lithium from the compromised water. This unexpected finding ignited their curiosity about the potential of these membranes to isolate lithium-6 from the more abundant lithium-7 isotopes present.
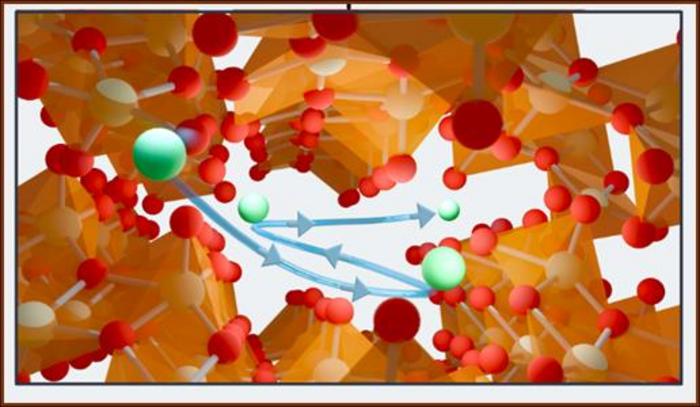
Central to the newfound method is a material known as zeta-vanadium oxide (ζ-V₂O₅), which is an inorganic compound engineered in the laboratory. This sophisticated compound showcases unique properties enabling it to attract lithium ions selectively due to its one-dimensional tunnel-like structural framework. Banerjee highlights that the zeta-V₂O₅ material’s remarkable characteristics not only lend themselves to energy storage applications, making it a phenomenal battery component, but also enhance its efficacy in isotope separation.
In a laboratory setup designed to evaluate the separation process, the research team constructed an electrochemical cell utilizing a zeta-V₂O₅ cathode. Upon introducing an aqueous solution containing lithium ions while applying electrical voltage, the cations migrated toward the negatively charged zeta-V₂O₅ matrix, penetrating into its intricate tunnels. The intrinsic differences in movement between lithium isotopes, due to their distinct masses, allow zeta-V₂O₅ to preferentially retain lithium-6 ions effectively.
The experimental results illustrate that lithium-6 ions adhere more robustly to the zeta-V₂O₅ tunnels compared to lithium-7 ions. As co-first author Andrew Ezazi explained, an analogy can be drawn using the tension of a spring—the lighter lithium-6 resonates in harmony with the bonds formed with vanadium oxide, while heavier lithium-7 is more prone to disrupt this connection, thus facilitating the absorption process. This nuanced mechanism of selectivity underscores the innovative shift away from toxic isolation methods.
As the process unfolds, an intriguing visual transformation occurs with the zeta-V₂O₅ compound—its color transitions from vibrant yellow to a dark olive green. This dramatic color change not only serves as an aesthetic marker of the lithium isolation process but also provides an essential visual cue for researchers monitoring the degree of lithium-6 enrichment over successive cycles, adding a layer of practicality to the method.
In their findings, the team reported that a single electrochemical cycle successfully enriched lithium-6 concentrations by approximately 5.7%. However, in order to obtain fusion-grade lithium—which necessitates a minimum of 30% lithium-6—the procedure requires repetition, with about 25 cycles needed for effective extraction. Interestingly, researchers projected that it may be possible to achieve an impressive 90% lithium-6 purity after roughly 45 cycles, showcasing a practical roadmap for escalating the efficiency of lithium separation processes.
While this discovery represents significant progress, Banerjee noted that current efforts do not yet extend to industrial-scale production of lithium-6. There remain substantial engineering challenges to negotiate, particularly in terms of optimizing the design of continuous flow systems that would facilitate the economical large-scale production of lithium-6 from seawater or contaminated sources. Nevertheless, preliminary results paint a promising picture, highlighting the potential for this technology to provide a low-cost source of fusion-grade lithium in the near future.
Additionally, the research team speculates that the principles governing the zeta-V₂O₅ material’s selective extraction capabilities could be extended to separate other isotopes—including those that are radioactive—from their non-radioactive counterparts. This broader scope of application reinforces the potential impact of their findings on various scientific fields, paving the way toward an array of innovative techniques for material separation.
As researchers prepare for the next steps, their focus shifts towards scaling the process for commercial viability. Banerjee expressed optimism regarding the future of nuclear fusion, suggesting that with the right support and collaboration, their findings could usher in a new era of clean energy production, unlocking the full potential of nuclear technology. The global interest in fusion energy has surged, propelling this research to prominence as the scientific community looks to translate findings into actionable solutions to environmental and energy crises.
In conclusion, the work conducted by Banerjee and his team stands as a testament to the powerful intersection of innovative material science and environmental sustainability. By challenging conventions and reimagining traditional processes, they have unlocked a method that could significantly influence the landscape of nuclear fusion and isotopic enrichment, all while sidestepping the health hazards associated with toxic materials. The pathway forward is laden with promise, and as excitement builds, the world watches and hopes for breakthroughs that could ultimately alter the very fabric of energy generation.
Subject of Research: Lithium-6 isotope enrichment and its isolation from lithium-7.
Article Title: Electrochemical 6-Lithium Isotope Enrichment Based on Insertion in 1D Tunnel-Structured V2O5.
News Publication Date: 20-Mar-2025.
Web References: 10.1016/j.chempr.2025.102486
References: Carrillo et al., “Electrochemical 6-Lithium Isotope Enrichment Based on Selective Insertion in 1D Tunnel-Structured V2O5”.
Image Credits: Harris Kohl and Andrew Ezazi.
Keywords
Nuclear fusion, Industrial production, Sustainable development, Chemical separation.
Tags: clean energy sourcesenvironmental impact of nuclear researchETH Zürich scientific innovationfuture of nuclear fusion energylithium-6 applications in energylithium-6 isotope isolationmercury-free lithium enrichmentnuclear fusion technologyOak Ridge National Laboratory researchsustainable nuclear energy solutionsTexas A&M University advancementsUS toxicity regulations