A groundbreaking study led by bioengineers from the University of California San Diego has unveiled significant sex-based differences in the progression of aortic valve stenosis (AVS), a serious heart valve disease. This research emphasizes how the genetic component of sex, specifically the presence of the Y chromosome, can alter the disease’s trajectory in males and females. Published on March 12 in the esteemed journal Science Advances, the findings highlight the critical need to consider biological sex in medical research and treatment strategies.
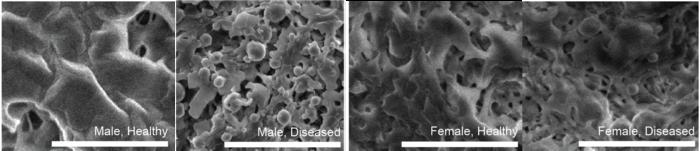
Heart valve disease, particularly aortic valve stenosis, poses a high risk of heart failure due to the stiffening of the heart’s aortic valve, which disrupts normal blood flow. This study has illuminated how males and females develop the disease through distinct biological pathways, revealing that the initial symptoms express differently based on sex. Males often experience calcium buildup in the valve at an earlier stage, while females face stiffening primarily due to fibrotic tissue formation. These insights not only deepen our understanding of the disease but also prompt the reconsideration of clinical practices that have traditionally overlooked such differences.
At the forefront of this research, senior author Brian Aguado, a professor at the UC San Diego Jacobs School of Engineering, articulates the importance of investigating how sex chromosomes can influence health outcomes. The study underscores the responsibility of the medical community to broaden their scope beyond conventional research that has historically favored male subjects. Aguado emphasizes that recognizing these differences is not just about identifying risks; it’s about enhancing treatments to ensure they are effective for everyone.
While historical medical research often focused predominantly on male populations, the tide is shifting as scientists and funding bodies advocate for a more nuanced understanding of sex and gender dynamics in health research. Several recent studies have revealed that accounting for sex differences can lead to more tailored and potentially more effective medical interventions. Aguado notes that ensuring inclusivity in research can significantly improve health outcomes and tailor treatment plans to individual patients.
The team discovered that a specific gene linked to the Y chromosome, known as UTY (ubiquitously transcribed tetratricopeptide repeat containing Y-linked), plays a critical role in driving the calcification of heart valves in males. This genetic link strengthens the notion that biological differences extend beyond mere reproductive organs and can impact the effectiveness of treatments based upon genetic predispositions.
In the early stages of AVS, heart valve cells undergo abnormal activation, leading to significant differences in how these cells behave in males versus females. In females, the activated cells predominantly transition to a myofibroblast state, contributing to valve stiffness through fibrosis. Conversely, in males, these cells have the propensity to differentiate further into bone-like cells, which generate calcium particles that lead to calcification. This is a vital distinction that can greatly influence future treatment protocols.
Utilizing advanced biomaterials, Aguado’s team engineered a hydrogel that simulates the natural microenvironment of aortic valve tissues. This setup allowed researchers to observe the behavior of heart valve cells in conditions that closely mimic their natural surroundings. In this engineered environment, they confirmed that male cells progressed towards calcification, while female cells showed a different response, providing critical evidence of the role environmental context plays in disease pathology.
The use of bioinspired materials reinforced the findings of sex-specific cellular responses to their environments. When housed within traditional Petri dishes, the significant differences between male and female cells dissipated, indicating that standard laboratory conditions can obscure vital biological variances. By creating conditions closer to those encountered by the cells in the human body, researchers can gain insights that have traditionally gone unnoticed.
Furthermore, the exploration of how the microenvironment influences the progression of AVS led to the incorporation of nanoparticles that signified calcification sites within the hydrogel. The presence of these nanoparticles intensified the differences observed in cellular responses based on sex, solidifying the hypothesis that environmental cues have profound implications on cell behavior and disease progression in aortic valve stenosis.
The implications of this research extend far beyond academic inquiry. As Aguado notes, identifying the specific mechanisms operated by the Y chromosome in disease progression opens new avenues for targeted therapies. The work serves as a foundation for future studies exploring drug combinations that can specifically interfere with the sex-disparate biological pathways leading to early-stage AVS.
Understanding the roles played by both the X and Y chromosomes in human health is only beginning to unfold. While much focus has been placed on the Y chromosome through this study, researchers like Gorashi suggest that equally significant pathways may exist on the X chromosome. Future investigations could yield a more comprehensive understanding of how genetics shape health outcomes.
In summary, this research not only sheds light on the critical influence of biological sex in determining disease progression in aortic valve stenosis but also advocates for a more inclusive approach in medical research. By acknowledging and studying these differences, the scientific community takes a step closer to developing personalized medical interventions that could significantly improve treatment effectiveness for all patients, irrespective of sex.
This study is a call to action for researchers and clinicians alike to recognize the importance of sex as a biological variable in disease processes. As greater emphasis is placed on understanding specific mechanisms that underpin these differences, the future of tailored medicine looks promising, hopeful for more equitable health outcomes in heart disease and beyond.
Subject of Research: Aortic Valve Stenosis and Sex Differences in Disease Progression
Article Title: Y chromosome–linked UTY modulates sex differences in valvular fibroblast methylation in response to nanoscale extracellular matrix cues
News Publication Date: March 13, 2025
Web References: Science Advances Article
References: Study detailed in Science Advances
Image Credits: Credit: Rayyan Gorashi
Keywords
Aortic Valve Stenosis, Sex Differences, Y Chromosome, UTY Gene, Calcium Buildup, Fibrosis, Bioengineering, Personalized Medicine, Heart Valve Disease, Clinical Outcomes
Tags: aortic valve stenosis progressionbiological sex in medical treatmentcalcium buildup in heart valvesclinical practices in heart diseasefibrotic tissue formation in femalesgenetic factors in heart valve diseaseheart failure risk factorsheart valve disease researchsex differences in disease symptomssex-based differences in heart diseaseUC San Diego bioengineering studyY chromosome influence on heart disease