In the ever-evolving landscape of synthetic chemistry, the development of robust molecular platforms has proved paramount in addressing the challenges posed by the synthesis of complex molecules. A significant advancement has been made by a research team from the Tokyo University of Science, led by Associate Professor Suguru Yoshida. Their study primarily focuses on a cutting-edge trivalent platform designed for triple click chemistry, which opens new avenues for constructing intricate molecular architectures. The trivalent platform features three distinct functional groups, effectively allowing for a controlled and selective approach to chemical synthesis.
Double and triple functionalization of molecular structures has traditionally presented scientists with a set of difficulties, especially when the molecular weight exceeds 1,000 daltons. A prime hurdle in this area often involves the lengthy and laborious processes required for synthesis, which detracts from efficiency and can lead to a proliferation of byproducts. However, the Yoshida team’s research emphasizes the utility of click chemistry as an essential toolkit that enables chemists to swiftly and efficiently construct larger and more complex structures. Their trivalent platform reflects a novel synergy between simplicity and efficiency, crucial in expediting chemical reactions while minimizing undesirable side reactions.
The foundations of this innovative research are grounded in “click chemistry,” a term that encapsulates a series of highly selective reactions. This approach facilitates the rapid assembly of smaller molecules into more complex arrangements. By capitalizing on the outstanding characteristics of click chemistry—its ability to enable precise reactions with minimal side products—researchers aim to forge new pathways to creating complex, target-specific compounds, particularly in medicinal chemistry and material science.
On the frontier of this research, the study of triple click chemistry emerges as a promising area. Trivalent platforms are designed specifically to house three different functional groups, each capable of undergoing selective reactions with various partners, promoting diversification in molecular synthesis. This innovative approach not only enhances the efficiency of creating complex molecules but also establishes the groundwork for exploring how these structures can interact within biological systems.
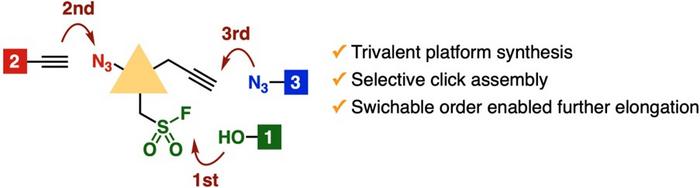
In their pursuit of advancing triple click chemistry, the Tokyo University of Science team meticulously synthesized a trivalent platform that boasts enhanced stability. This stability arises from a longer linker integrated within the central scaffold, allowing a multitude of reactions to be performed sequentially. This is of particular importance as it empowers chemists to target each distinct functional moiety without destabilizing the integral structure, thus preserving the integrity of the synthesized complex molecules.
The Yoshida research team meticulously detailed their methods in a recent publication. The sequential targeting of each functional group was showcased through various reactions, including the sulfur-fluoride exchange, which allowed them to generate alcohols from the fluorosulfonyl moiety. This reaction was executed with notable efficiency, yielding high quantities of desired products while maintaining the reactivity of the azide and alkyne moieties intact. This exemplifies the versatility and efficiency of the developed platform, as chemists can select reaction conditions to optimize yields according to specific needs.
Following the success of the alcohol synthesis, further transformations were applied to the azide moiety. The research team conducted a series of well-established reactions such as copper-catalyzed azide-alkyne cycloaddition and strain-promoted azide-alkyne cycloaddition. These types of transformations are particularly noteworthy within the field of organic chemistry, as they facilitate the formation of triazole compounds—an essential class of compounds in pharmaceuticals and bioengineering.
Another interesting facet of this research is how the order of these transformations can vary without resulting in detrimental effects on the triazole formation. Selective click reactions were shown to yield triazoles irrespective of the original sequence of targeting each moiety. This flexibility highlights the resilience of the trivalent platform, making it an efficient tool for chemists who require adaptability in their synthetic pathways. The implications of this flexibility could redefine the strategies employed in pharmaceutical development and compound library generation.
As the researchers probed further into the capabilities of the trivalent platform, they unveiled significant insights into the synthesis of complex triazoles through straightforward one-pot reactions. The critical takeaway here is the ability of the platform to yield multifunctional molecules via a streamlined approach, reducing the time and labor typically associated with traditional synthesis techniques. This is particularly significant when considering the profound demands placed on research teams striving for efficiency in the modern landscape of scientific inquiry.
This research advances sustainability in synthetic chemistry by utilizing simpler materials rather than complex precursors. The implications are profound, as this simplification not only accelerates the research process but also aligns with the broader goals of eco-friendly chemistry practices. This approach is essential in fostering developments that contribute positively to pharmaceutical science and even agricultural sectors, potentially leading to remarkable innovations in drug delivery systems and other health-related applications.
The pursuit of sustainable methods in synthetic chemistry becomes increasingly aligned with global efforts toward greener practices. The trivalent platforms developed by Dr. Yoshida and his team not only promote efficiency in molecular synthesis but also serve as pivotal components for further research aimed at environmental preservation, fostering collaborations that further the United Nations Sustainable Development Goals (SDGs). Their endeavor illustrates the interconnectedness of chemistry and sustainable innovation, aiming to revolutionize how we approach both scientific inquiry and real-world applications.
Looking ahead, the research team at Tokyo University of Science underscores an overarching ambition: to create new molecules that could revolutionize life sciences. The goal reflects a commitment not just to academic excellence but to the application of science that harmonizes with the needs of society and the environment. In this quest, the newly developed trivalent platform is seen as a versatile tool that has the potential to contribute to groundbreaking advancements in medicine, materials science, and beyond.
Overall, the significance of this research extends well beyond academic interest. It heralds a future where functionalized, multi-triazoles can be synthesized with unprecedented ease and efficiency. This paves the way for new therapeutic agents, innovative materials, and cutting-edge applications in biotechnology. The commitment from both the research community and the Tokyo University of Science to pursue this path will undoubtedly yield fruitful results, establishing new benchmarks in the field of synthetic organic chemistry.
As we reflect upon the groundbreaking work emerging from TUS, the implications of adopting this new synthetic chemistry approach extend to the healthcare sector. The potential to tackle intractable diseases through innovative drug development routes that rely on these versatile trivalent platforms underscores the urgency and importance of this research. The ongoing exploration into the realms of click chemistry through this innovative platform will likely yield numerous other discoveries that will continue to propel the fields of medicine, environmental science, and engineering forward in exciting new directions.
Subject of Research: Triple Click Chemistry Using Trivalent Platforms
Article Title: Three-step click assembly using trivalent platforms bearing azido, ethynyl, and fluorosulfonyl groups
News Publication Date: January 7, 2025
Web References: Chemical Communications
References: DOI 10.1039/D4CC06585A
Image Credits: Credit: Dr. Suguru Yoshida from Tokyo University of Science, Japan
Keywords: Click chemistry, Trivalent platforms, Organic synthesis, Pharmaceutical development, Sustainable chemistry, Drug design, Chemical biology, Bioengineering, Medicinal chemistry.
Tags: advancements in synthetic chemistryclick chemistry in drug developmentefficient construction of complex moleculesinnovative molecular architecturesminimizing byproducts in chemical reactionsovercoming challenges in molecular functionalizationrapid synthesis of large moleculesselective chemical synthesis techniquesSuguru Yoshida’s contributions to chemistryTokyo University of Science researchtransformative techniques in drug discoverytrivalent platform for molecular synthesis