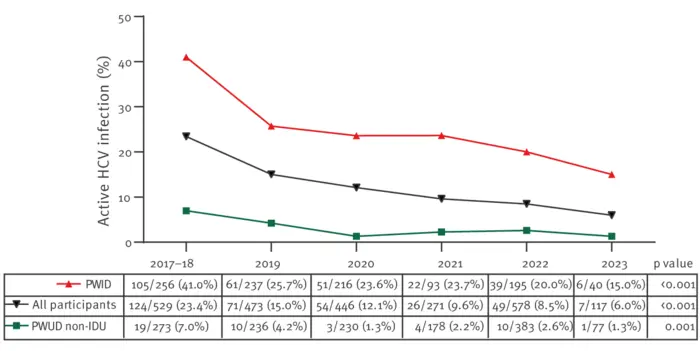
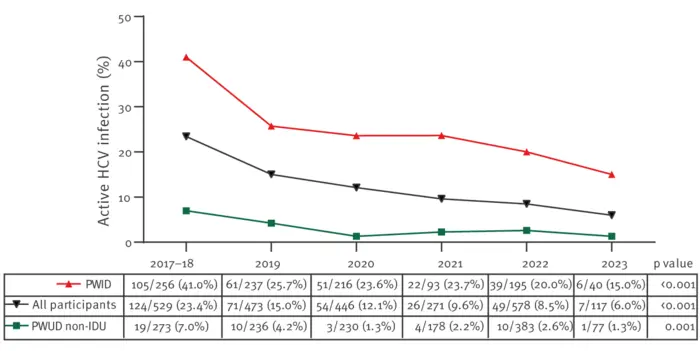
A study conducted through a mobile screening unit in Madrid, Spain from 2017 to 2023 and published in Eurosurveillance found that active hepatitis C virus (HCV) infection decreased from 23% to 6% in that period among people who use drugs (PWUD) that visited the unit. The study found that the use of intravenous drugs was the most significant risk factor for infection among PWUD. It confirmed that HCV screening and treatment programmes targeting this at-risk population are effective and can help achieve the World Health Organization goal of HCV elimination as public health threat by 2030.
Credit: Eurosurveillance
A study conducted through a mobile screening unit in Madrid, Spain from 2017 to 2023 and published in Eurosurveillance found that active hepatitis C virus (HCV) infection decreased from 23% to 6% in that period among people who use drugs (PWUD) that visited the unit. The study found that the use of intravenous drugs was the most significant risk factor for infection among PWUD. It confirmed that HCV screening and treatment programmes targeting this at-risk population are effective and can help achieve the World Health Organization goal of HCV elimination as public health threat by 2030.
Study participants and methods
Participants were recruited in ‘hotspots’ in Madrid where high-risk individuals gathered for dealing and drug consumption. The study included individuals over 18 and with active drug use in the year before being screened.
A mobile screening unit approached these ‘hotspots’’. A nurse collected blood samples from participants for HCV testing, and investigators gathered data on demographic, epidemiology, substance use and sexual risk behaviour.
Results and public health implications
2 349 participants of the initial cohort of 5 270 were actively using drugs. Of these, 2 264 (43%) underwent an HCV antibody test, with 195 (8.6%) participating more than once. Among the group who were tested, 685 (13%) tested positive for anti-HCV antibodies. Of those, 605 participants agreed to undergo HCV-RNA testing, with an active HCV infection detected in 314 (51.9%) individuals.
Participants who reported using drugs and with an active HCV infection were significantly older and were more likely to be of European (Western Europe and Eastern Europe) origin, homeless, consuming cocaine and heroin, injecting drugs, and undergoing opioid substitution treatment than participants who used drugs without an active HCV infection. Significantly, the use of a mobile screening unit led almost 70% of participants who tested positive to start treatment.
The study observed a strong decrease in active HCV infection across the entire population from 23.4% in 2017 to 6% in 2023. However, the prevalence remains 30 and 70 times higher among people who use drugs and people who inject drugs, respectively, than in the general population.
The decrease could be attributed to measures that have helped users of intravenous drugs overcome barriers to testing and treatment, such as better access to testing and care through decentralised approaches, simplified one-step diagnosis, and fewer treatment restrictions. Public health campaigns have also helped raise awareness of the risks related to intravenous drug use.
Additional outputs on hepatitis
Ahead of World Hepatitis Day, Eurosurveillance has also published a study conducted in Australia demonstrating the feasibility and effectiveness of using surveillance systems to improve access to treatment and care of hepatitis C. Additionally, it has published research on the prevalence of chronic hepatitis C infections in Estonia and Romania, with levels shown to be low in both countries, as well as an accompanying editorial highlighting steps needed to achieve the goal of eliminating hepatitis C as public health threat by 2030.
Journal
Eurosurveillance
DOI
10.2807/1560-7917.ES.2024.29.29.2300712
Method of Research
Observational study
Subject of Research
People
Article Title
Decrease in active hepatitis C infection among people who use drugs in Madrid, Spain, 2017 to 2023: a retrospective study
Article Publication Date
18-Jul-2024
COI Statement
None declared.