Palo Alto, CA, June 26, 2024 – In a leap forward for genetic engineering, a team of researchers from the Arc Institute have discovered the bridge recombinase mechanism, a precise and powerful tool to recombine and rearrange DNA in a programmable way.
Credit: Visual Science
Palo Alto, CA, June 26, 2024 – In a leap forward for genetic engineering, a team of researchers from the Arc Institute have discovered the bridge recombinase mechanism, a precise and powerful tool to recombine and rearrange DNA in a programmable way.
The study published today in Nature reports their discovery of the first DNA recombinase that uses a non-coding RNA for sequence-specific selection of target and donor DNA molecules. This bridge RNA is programmable, allowing the user to specify any desired genomic target sequence and any donor DNA molecule to be inserted.
“The bridge RNA system is a fundamentally new mechanism for biological programming,” said Hsu, senior author of the study and an Arc Institute Core Investigator and UC Berkeley Assistant Professor of Bioengineering. “Bridge recombination can universally modify genetic material through sequence-specific insertion, excision, inversion, and more, enabling a word processor for the living genome beyond CRISPR.”
Arc senior scientist Matthew Durrant and UC Berkeley bioengineering graduate student Nick Perry were the lead authors of the discovery. The research was developed in collaboration with the labs of Silvana Konermann, Arc Institute Core Investigator and Stanford University Assistant Professor of Biochemistry, and Hiroshi Nishimasu, Professor of Structural Biology at the University of Tokyo.
Programmable RNA
The bridge recombination system hails from insertion sequence 110 (IS110) elements, one of countless types of transposable elements – or “jumping genes” – that cut and paste themselves to move within and between microbial genomes. Transposable elements are found across all life forms, and have evolved into professional DNA manipulation machines in order to survive. The IS110 elements are very minimal, consisting only of a gene encoding the recombinase enzyme, plus flanking DNA segments that have, until now, remained a mystery.
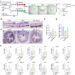
The Hsu lab found that when IS110 excises itself from a genome, the non-coding DNA ends are joined together to produce an RNA molecule – the bridge RNA – that folds into two loops. One loop binds to the IS110 element itself, while the other loop binds to the target DNA where the element will be inserted. The bridge RNA is the first example of a bispecific guide molecule, specifying the sequence of both target and donor DNA through base-pairing interactions.
Each loop of the bridge RNA is independently programmable, allowing researchers to mix and match any target and donor DNA sequences of interest. This means the system can go far beyond its natural role that inserts the IS110 element itself, instead enabling insertion of any desirable genetic cargo—like a functional copy of a faulty, disease-causing gene—into any genomic location. In this work, the team demonstrated over 60% insertion efficiency of a desired gene in E. coli with over 94% specificity for the correct genomic location.
“These programmable bridge RNAs distinguish IS110 from other known recombinases, which lack an RNA component and cannot be programmed,” said graduate student Nick Perry. “It’s as if the bridge RNA were a universal power adapter that makes IS110 compatible with any outlet.”
The Hsu lab’s discovery is complemented by their collaboration with the lab of Dr. Hiroshi Nishimasu at the University of Tokyo, also published today in Nature. The Nishimasu lab used cryo-electron microscopy to determine the molecular structures of the recombinase-bridge RNA complex bound to target and donor DNA, sequentially progressing through the key steps of the recombination process.
With further exploration and development, the bridge mechanism promises to usher in a third generation of RNA-guided systems, expanding beyond the DNA and RNA cutting mechanisms of CRISPR and RNA interference (RNAi) to offer a unified mechanism for programmable DNA rearrangements. Critical for the further development of the bridge recombination system for mammalian genome design, the bridge recombinase joins both DNA strands without releasing cut DNA fragments – sidestepping a key limitation of current state-of-the-art genome editing technologies.
“The bridge recombination mechanism solves some of the most fundamental challenges facing other methods of genome editing,” said research co-lead Durrant. “The ability to programmably rearrange any two DNA molecules opens the door to breakthroughs in genome design.”
Read the papers in Nature here and here. A short video of the discovery, developed in collaboration with Visual Science, is available here.
Other co-authors include James Pai and Aditya Jangid (Arc Institute and University of California, Berkeley); Januka Athukoralage, John McSpedon and April Pawluk (Arc Institute); and Masahiro Hiraizumi (University of Tokyo). This work was supported by the Arc Institute, the NIH Biology and Biotechnology of Cell and Gene Therapy Training Program, JSPS KAKENHI Grant Numbers 21H05281 and 22H00403; Takeda Medical Research Foundation; the Inamori Research Institute for Science, Rainwater Foundation, Curci Foundation, Rose Hill Innovators Program, S. Altman, V. and N. Khosla, and anonymous gifts to the Hsu Lab.
—
About Arc Institute
Arc Institute is an independent nonprofit research organization located in Palo Alto, California that aims to accelerate scientific progress and understand the root causes of complex diseases. Arc’s model gives scientists complete freedom to pursue curiosity-driven research agendas and fosters deep interdisciplinary collaboration. The Institute operates in close partnership with Stanford University, the University of California, Berkeley, and the University of California, San Francisco. Arc was founded in 2021 by Silvana Konermann, Patrick Hsu, and Patrick Collison.
Journal
Nature
DOI
10.1038/s41586-024-07552-4
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Bridge RNAs direct programmable recombination of target and donor DNA
Article Publication Date
26-Jun-2024
COI Statement
P.D.H. acknowledges outside interest in Stylus Medicine, Spotlight Therapeutics, Circle Labs, Arbor Biosciences, Varda Space, Vial Health, and Veda Bio, where he holds various roles including as co-founder, director, scientific advisory board member, or consultant. M.G.D. acknowledges outside interest in Stylus Medicine. P.D.H., M.G.D., N.T.P., S.K., J.S.A., M.H. and H.N. are inventors on patents relating to this work. The remaining authors declare no competing interests.