The sweet smell of strawberries and other fruits is thanks to a chemical compound called ester, which is also found in many fats and polyesters. The ubiquitous compound can be broken down to produce desirable alcohols and other chemicals for use across industries including pharmaceuticals and cosmetics, but the process can be costly, both financially and in terms of the environment.
Credit: Shintaro Okumura
The sweet smell of strawberries and other fruits is thanks to a chemical compound called ester, which is also found in many fats and polyesters. The ubiquitous compound can be broken down to produce desirable alcohols and other chemicals for use across industries including pharmaceuticals and cosmetics, but the process can be costly, both financially and in terms of the environment.
Now, a team of researchers with the National Institutes of Natural Sciences (NINS) in Japan has developed a novel approach using light as an energy source. They published their findings on June 14 in the Journal of the American Chemical Society.
In a seemingly counterintuitive move, to break down — or reduce, in the chemical parlay — esters, scientists actually add electrons to the compound. The addition of electrons forces the groups comprising the ester to reduce to more basic components. The conventional methods for ester reduction require excess amount of highly reactive and difficult to handle metal reductants.
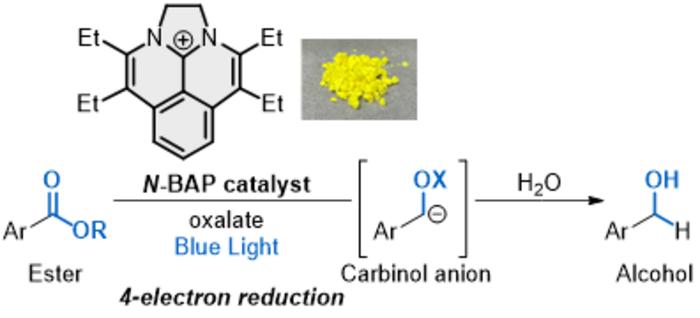
Now, researchers are investigating the use of sustainable photocatalysts. Photocatalysts or catalysts that activate when excited by light, are known to promote an electron transfer process between the catalyst and organic compounds without using the highly reactive metal reductants. Conventional photocatalysts, involving expensive and non-renewable noble metals, reduces the limited organic compounds, and typically add only one electron to the compounds. Called single-electron transfer (SET), the process must proceed multiple times until the desired number of electrons are added to achieve the target reduction of esters.
“During the last decade, photocatalytic reactions have gained significant attention as desirable methods suitable for the United Nations’ Sustainable Development Goals (SDGs) in organic synthesis,” said co-corresponding author Shintaro Okumura, assistant professor at the Institute for Molecular Science (IMS) of NINS. “Photocatalysts promote redox reactions using visible light as an energy source in the absence of metal reductants. However, photocatalytic reactions through a multielectron transfer process have been less developed, so photocatalytic reduction of esters to form alcohols, which requires four electrons, has remained undeveloped. The photocatalytic reduction of esters to form alcohols is a formidable challenge because it requires an unprecedented successive quadruple SET process,” Okumura said.
To achieve this quadruple SET process, the researchers developed a novel photocatalyst they dubbed “N-BAP.” When irradiated with blue light, the photocatalyst initiates the reaction to produce a chemical group that reacts with water and a second carbon-based chemical group. With the addition of oxalate, a negatively charged molecule found widely in nature, the reaction can add four electrons in quick succession — resulting in the desired alcohols.
“The combination of N-BAP catalyst with oxalate as a traceless reductant permits the rapidly sequential four-electron reduction of esters to generate carbinol anions, with subsequent protonation to give alcohols,” Okumura said. “This work could pave the novel transformation of esters and is expected to contribute to a sustainable society as a green organic synthesis suitable for the SDGs.”
Okumura is also affiliated with. Other contributors include co-corresponding author Yasuhiro Uozumi, Shusuke Hattori and Lisa Fang, all affiliated with IMS. Okumura, Uozumi and Hattori are also affiliated with the Department of Functional Molecular Science at The Graduate University for Advanced Studies, also known as SOKENDAI.
The Japan Science and Technology Agency and the Japan Society for the Promotion of Science funded this research.
Information of the paper:
Authors: Shintaro Okumura, Shusuke Hattori, Lisa Fang, Yasuhiro Uozumi
Journal Name: Journal of the American Chemical Society
Journal Title: “Multielectron Reduction of Esters by a Diazabenzacenaphthenium Photoredox Catalyst”
DOI: 10.1021/jacs.4c05272 (open access)
Financial Supports:
ACT-X, Japan Science and Technology Agency: JPMJAX23D6
Grant-in-Aid for Transformative Research Areas(A): JP24H01873
Grant-in-Aid for Early-Career Scientists: JP24K17688
Contact Person:
Shintaro Okumura
TEL/FAX: +81-75-383-2753 / +81-75-383-2748-
E-mail: okumura.shintaro.6e_at_kyoto-u.ac.jp (Please replace the “_at_” with @)
Yasuhiro Uozumi
TEL/FAX: +81-564-59-5571 / +81-564-59-5574}
E-mail: uo_at_ims.ac.jp (Please replace the “_at_” with @)
Journal
Journal of the American Chemical Society
DOI
10.1021/jacs.4c05272
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Multielectron Reduction of Esters by a Diazabenzacenaphthenium Photoredox Catalyst
Article Publication Date
14-Jun-2024




