As embryos grow from conception to birth, cells multiply rapidly and move in a highly organized manner to create the skeleton, organs and other crucial systems. But how do cells know to move in precisely the right direction at the right time to create a fully formed, complex living organism? This is a deeply challenging question for scientists.
Credit: UC San Diego
As embryos grow from conception to birth, cells multiply rapidly and move in a highly organized manner to create the skeleton, organs and other crucial systems. But how do cells know to move in precisely the right direction at the right time to create a fully formed, complex living organism? This is a deeply challenging question for scientists.
To help uncover the answer, University of California San Diego Assistant Professor of Physics Mattia Serra and colleagues at Politecnico di Milano (Italy) have developed a new method that can manipulate the movement of embryonic cells using short-time attractors — a concept Serra had previously developed and adopted to help search and rescue operations at sea. Their work appears in Physical Review Letters.
Short-time attractors are structures that influence a system’s dynamics and motion for a limited time, but do not determine long-term behaviors. By modulating the spatial distribution of myosin — the molecular motor that drives cell movement — the researchers were able to control the positioning of these attractors, directing cell accumulation to targeted areas of the embryo.
While myosin drives cell movement within the embryo, there are also external forces, or disturbances, that push and pull against the embryo as well. These disturbances are imposed on rather than controlled by the embryo.
This is a delicate dance. The embryo must optimally distribute myosin so that cells move toward the attractors necessary for development while also contending with the imposed disturbances.
Using theory and simulations, the researchers were able to devise an optimal control strategy to create and steer short-time attractors in flows similar to those found in embryo development.
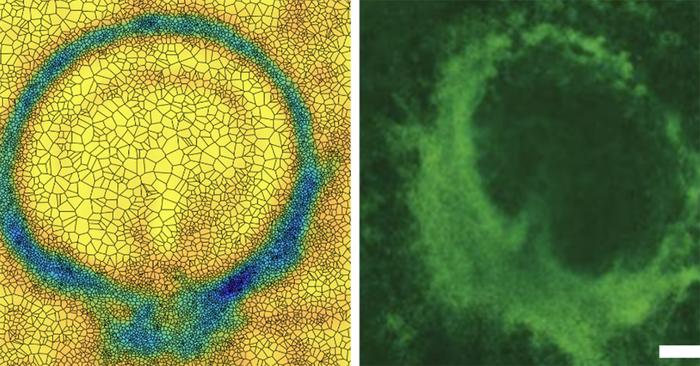
To confirm their theory, collaborators in the Weijer group at the University of Dundee (Scotland) manipulated the myosin distribution of a chick embryo. Normally, the embryo develops a short-time attractor as a line — this is where the main body axis forms. Serra’s predictions suggested that with a particular distribution of myosin, they could make a ring-shaped short-time attractor. The Weijer group was able to implement the suggested myosin distribution in a living embryo, and it developed a circular attractor rather than a linear one.
This new method of controlling cell flows can be used in engineering synthetic organs and organoids and may help in regenerative medicine applications.
“Multicellular flows are complex and can be overwhelming to study. Attractors and repellers compress this complexity into its essential units, that can be controlled and used to unravel the underlying principles driving multicellular flows,” stated Serra.
Full list of authors: Mattia Serra (UC San Diego), Carlo Sinigaglia and Francesco Braghin (both Politecnico di Milano).
Journal
Physical Review Letters
DOI
10.1103/PhysRevLett.132.218302
Method of Research
Experimental study
Subject of Research
Animal tissue samples
Article Title
Optimal Control of Short-Time Attractors in Active Nematics
Article Publication Date
22-May-2024




