LA JOLLA, CA—Scripps Research chemists have accomplished a long elusive feat in synthetic chemistry: the invention of a broadly useful method for constructing “gamma chiral centers” on simple starting compounds called carboxylic acids. The method, published on May 16, 2024 in Science, significantly extends the ability of chemists to build and modify complex pharmaceutical molecules and other valuable chemical products.
Credit: Scripps Research
LA JOLLA, CA—Scripps Research chemists have accomplished a long elusive feat in synthetic chemistry: the invention of a broadly useful method for constructing “gamma chiral centers” on simple starting compounds called carboxylic acids. The method, published on May 16, 2024 in Science, significantly extends the ability of chemists to build and modify complex pharmaceutical molecules and other valuable chemical products.
The term chiral refers to a type of asymmetry that allows some chemical compounds to exist in left-handed and right-handed forms. Often, only one of these forms has the desired biochemical activity, but for synthetic chemists, stereoselective reactions—those that yield just the desired form—are almost always challenging.
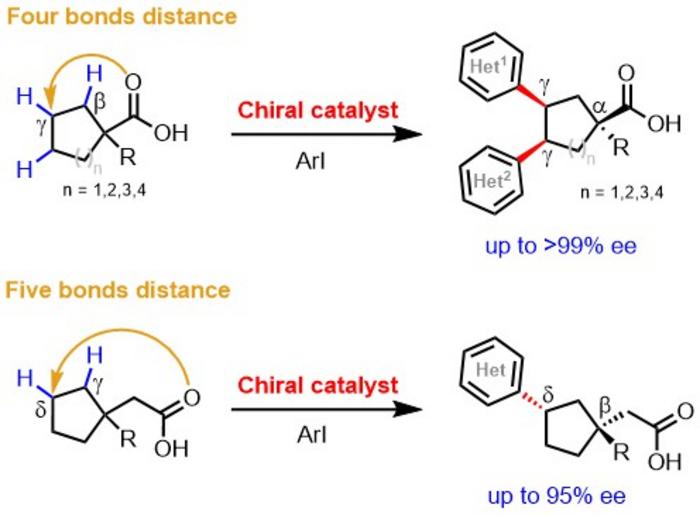
The new method enables what had been impossible except in narrow cases: creating a center of chiral asymmetry at a hard-to-reach position called the “gamma” position on a cyclic carboxylic acid.
“This approach offers unprecedented and relatively easy access to a broad set of chiral carbocycles that are privileged structures for pharma industry drug discovery programs,” says study senior author Jin-Quan Yu, PhD, Frank and Bertha Hupp Professor of Chemistry and Bristol Myers Squibb Endowed Chair in Chemistry at Scripps Research.
The co-first authors were Tao Zhang, PhD, and Zi-Yu Zhang, PhD, both postdoctoral research associates in the Yu lab.
A potentially valuable but elusive method
The development of small molecule pharmaceuticals or other chemical products typically involves the construction of many hundreds or thousands of compounds, each representing a variation on a central structural theme. Once these “libraries” of compounds are constructed, they are methodically tested for the desired biological or chemical activity; in this way, developers can zero in on the best compound for further refinement. Naturally, in constructing these libraries and in making more refined variants, chemists would like to have easy and versatile techniques for building and modifying molecules. But while their tools have greatly improved over the years, some types of molecular transformation have remained essentially undoable, despite the obvious value they would have. Gamma chiral center construction using readily available carboxylic acids has been one of these, defying the efforts of prominent synthetic chemistry labs.
The Yu lab’s success hinged on the development of special “ligand” molecules containing both oxazoline and pyridone structural elements. Ligand molecules help bring the reaction catalyst to the right spot on the initial compound. In this case, they fasten to one point on the starting carboxylic acid—which contains a ring of mostly carbon atoms—and direct a bond-breaking palladium atom to the distant gamma position on the other side of the ring. The effect is to remove a hydrogen atom from the backbone carbon atom at the targeted spot, allowing a new cluster of atoms to bond to the carbon—thus adding complexity to the molecule in a precise way.
This type of reaction is called a “C-H activation,” and over the past decade, Yu and his team have reported similar C-H activation methods for constructing chiral centers at “alpha” and “beta” positions on carboxylic acids.
The chemists demonstrated the power and versatility of their new method by using it to make gamma chiral centers on a wide variety of relatively simple carboxylic acid starting compounds containing rings of from five to eight carbon atoms. In one case, they achieved a single-step synthesis of a chiral version of a cancer drug molecule called an HDAC inhibitor—whose standard, patented synthesis method requires 10 steps and a costly separation to obtain pure samples of the left-handed or right-handed form.
The team also used the new technique to add complexity to existing drug molecules, including the steroid hormone pregnenolone.
Finally, the chemists showed they could use their new approach sequentially on a starting molecule to construct three chiral centers—including a very challenging “delta” chiral center.
The Yu lab is now working to extend the new approach so it can be used for making other types of chiral molecules.
“Enantioselective Remote Methylene C−H (Hetero)Arylation of Cycloalkane Carboxylic Acids” was co-authored by Tao Zhang, Zi-Yu Zhang, Guowei Kang, Tao Sheng, Jie-Lun Yan, Yuan-Bin Yang, Yuxin Ouyang and Jin-Quan Yu, all of Scripps Research.
Support for the research was provided by the National Institutes of Health (2R01GM084019).
About Scripps Research
Scripps Research is an independent, nonprofit biomedical institute ranked one of the most influential in the world for its impact on innovation by Nature Index. We are advancing human health through profound discoveries that address pressing medical concerns around the globe. Our drug discovery and development division, Calibr, works hand-in-hand with scientists across disciplines to bring new medicines to patients as quickly and efficiently as possible, while teams at Scripps Research Translational Institute harness genomics, digital medicine and cutting-edge informatics to understand individual health and render more effective healthcare. Scripps Research also trains the next generation of leading scientists at our Skaggs Graduate School, consistently named among the top 10 US programs for chemistry and biological sciences. Learn more at www.scripps.edu.
Journal
Science
DOI
10.1126/science.ado1246
Article Title
Enantioselective remote methylene C–H (hetero)arylation of cycloalkane carboxylic acids
Article Publication Date
17-May-2024




