Post-mitotic neurons in the brain that re-enter the cell cycle quickly succumb to senescence, and this re-entry is more common in Alzheimer’s disease, according to a new study published April 9th in the open-access journal PLOS Biology by Kim Hai-Man Chow and colleagues at the Chinese University of Hong Kong. The phenomenon may provide an opportunity to learn more about the neurodegeneration process, and the technique used to make this discovery is readily applicable to other inquiries about unique populations of cells in the brain.
Credit: Kim Hei-Man Chow (CC-BY 4.0, https://creativecommons.org/licenses/by/4.0/)
Post-mitotic neurons in the brain that re-enter the cell cycle quickly succumb to senescence, and this re-entry is more common in Alzheimer’s disease, according to a new study published April 9th in the open-access journal PLOS Biology by Kim Hai-Man Chow and colleagues at the Chinese University of Hong Kong. The phenomenon may provide an opportunity to learn more about the neurodegeneration process, and the technique used to make this discovery is readily applicable to other inquiries about unique populations of cells in the brain.
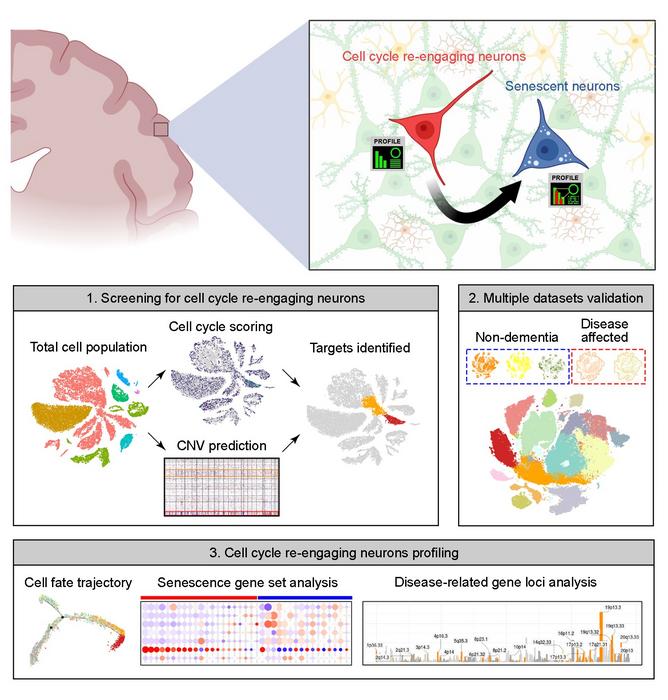
Most neurons in the brain are post-mitotic, meaning they have ceased to divide. For many years, it had been assumed that this post-mitotic state was permanent. Recent discoveries have shown that a small proportion of neurons re-enter the cell cycle, but little is known about their fate after they do.
To address this question, the authors turned to publicly accessible databases of “snRNA-seq” data, in which individual single nuclei are isolated and their RNA is sequenced, providing a snapshot of what a cell was doing at the time of isolation. The cell cycle proceeds through distinct phases, including growth, DNA synthesis, division-specific growth, and mitosis, and each phase is characterized by a specific set of proteins required to carry it out. This allowed the authors to use the set of RNAs to tell them which phase of the cycle any specific nucleus was in.
Their data included information on over 30,000 nuclei, each of which was assigned a score based on the level of expression of a set of about 350 cell cycle-related genes. They found that small populations of excitatory neurons had indeed re-entered the cell cycle. These cells did not, for the most part, continue successfully through the cell cycle to produce daughter neurons, however. Instead, cells undergoing re-entry also had elevated expression of genes associated with senescence; in effect, the cells had reawakened only to enter senescence.
Intriguingly, the authors found that neurons in the brains of Alzheimer’s disease patients reentered the cell cycle at a higher rate, and that those neurons that had reentered the cell cycle and aged had increased expression of multiple genes associated with a higher risk of Alzheimer’s disease, including those that contribute directly to production of amyloid, the sticky protein that aggregates in the AD brain. Similarly, brains from patients with Parkinson’s disease and Lewy body dementia had an increase in the proportion of re-entering neurons compared to healthy brains.
The neurobiological significance of this heightened re-entry for the diseased brain is still unclear, but the analytical approach taken here may offer deeper insights into neuronal subpopulations within the brain, as well as shedding light on disease mechanisms in neurodegenerative diseases.
“Because of the rare existence and random localization of these cells in the brain, their molecular profiles and disease-specific heterogeneities remain unclear,” Chow said. “While experimental validations of these findings in relevant human samples will be conducted in the future, the applicability of this analytical approach in different diseases and cross-species settings offers new opportunities and insights to supplement mainstay histological-based approaches in studying the roles of these cells in brain aging and disease pathogenesis.”
The authors add, “This bioinformatics analytical pipeline demonstrated will offer the field a new tool to unbiasedly dissect cell cycle re-engaging and senescent neurons, and to dissect their heterogeneities in healthy versus disease-affected brains.”
#####
In your coverage, please use this URL to provide access to the freely available paper in PLOS Biology: http://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3002559
Citation: Wu D, Sun JK-L, Chow KH-M (2024) Neuronal cell cycle reentry events in the aging brain are more prevalent in neurodegeneration and lead to cellular senescence. PLoS Biol 22(4): e3002559. https://doi.org/10.1371/journal.pbio.3002559
Funding: The work was supported, in part, by grants from the following: The Hong Kong Research Grants Council (RGC)-General Research Fund (GRF) (PI: ECS24107121, GRF16100219 and GRF16100718) (all to K.H-M.C) and the RGC- Collaborative Research Fund (CRF) (Co-I: C4033-19EF) (K.H-M.C); the National NaturalScience Foundation-Excellent Young Scientists Fund 2020 (Ref: 32022087) (K.H-M.C); Alzheimer’s Association Research Fellowship (PI: AARF-17-531566) (K.H-M.C). All the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Journal
PLoS Biology
DOI
10.1371/journal.pbio.3002559
Method of Research
Observational study
Subject of Research
Cells
COI Statement
Competing interests: The authors have declared that no competing interests exist.




