In cellular biology, unraveling the complexities of cellular function at the molecular level remains a paramount endeavor. Significant scientific focus has been placed on understanding the interactions at organelle contact sites, especially between mitochondria and the endoplasmic reticulum (ER). These sites are critical hubs for the exchange of essential biomolecules, such as lipids and calcium, which are vital for maintaining cellular homeostasis. Disruptions in this inter-organelle communication are implicated in the onset of various diseases, including neurodegenerative disorders, emphasizing the need to elucidate the mechanisms governing organelle interactions. However, the study of these dynamic complexes presents significant challenges due to the lack of available tools, complicating the quest to understand ER-mitochondria contact sites.
Credit: POSTECH
In cellular biology, unraveling the complexities of cellular function at the molecular level remains a paramount endeavor. Significant scientific focus has been placed on understanding the interactions at organelle contact sites, especially between mitochondria and the endoplasmic reticulum (ER). These sites are critical hubs for the exchange of essential biomolecules, such as lipids and calcium, which are vital for maintaining cellular homeostasis. Disruptions in this inter-organelle communication are implicated in the onset of various diseases, including neurodegenerative disorders, emphasizing the need to elucidate the mechanisms governing organelle interactions. However, the study of these dynamic complexes presents significant challenges due to the lack of available tools, complicating the quest to understand ER-mitochondria contact sites.
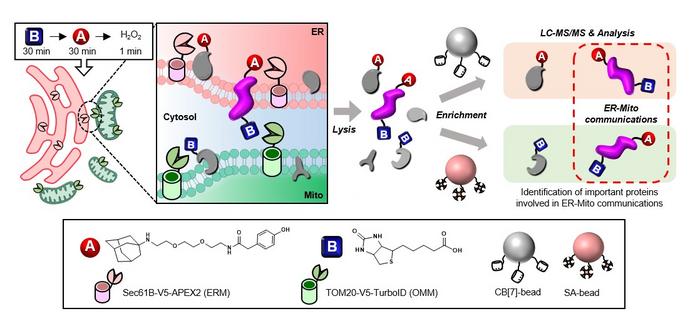
Emerging from this need, a novel strategy called “OrthoID” has been developed through the collaborative efforts of scientists from POSTECH, Daegu Catholic University School of Medicine, and Seoul National University. Featured in Nature Communications, OrthoID addresses this challenge by refining our ability to identify proteins that act as mediators in these critical conversations.
Traditional methods relied heavily on the streptavidin-biotin (SA-BT) binding pair system, derived from nature, for tagging and isolating these mediator proteins. However, this approach has its limitations, particularly in capturing the full spectrum of protein interactions between two different organelles. OrthoID overcomes these limitations by introducing an additional synthetic binding pair, cucurbit[7]uril-adamantane (CB[7]-Ad), to work alongside SA-BT. The combination of mutually orthogonal binding pair systems allowed a more precise identification and analysis of the mediator proteins that freely translocate between the ER and mitochondria, facilitating a deeper exploration of the proteins involved in the organelle contact sites and uncovering their roles in cellular functions and disease mechanisms.
Through meticulous experiments, the researchers have demonstrated the efficacy of OrthoID in rapidly and accurately labeling proteins involved in the dynamic processes of organelle communication. By leveraging proximity labeling techniques (APEX2 and TurboID) with orthogonal binding pair systems, the method effectively labeled and isolated proteins facilitating the critical interactions between mitochondria and ER. This approach not only identifies known proteins involved in ER-mitochondria contacts but also uncovers new protein candidates, including LRC59, whose roles at the contact site were previously unknown. Moreover, they also successfully pinpointed the multiple protein sets undergoing structural and locational changes at the ER-mitochondria junction during critical cellular process such as mitophagy, where damaged mitochondria are targeted for degradation.
“The flexibility and modularity of OrthoID are among its greates strengths.” states Prof. Kimoon Kim who led the research from POSTECH. This adaptability not only allows for the study of various organelle contact sites but also opens new avenues for exploring complex cellular communications, overcoming the technical limitations of existing methods.”
Prof. Kyeng Min Park from Daegu Catholic University School of Medicine adds, “OrthoID stands as a versatile and useful research tool, aimed to decode the complex language of cellular communication. It is expected to facilitate discoveries that will have profound implications for understanding cellular health, elucidating disease mechanisms, and fostering the development of new therapeutic strategies.”
The collaborative team included Prof. Kimoon Kim and Dr. Ara Lee from the Department of Chemistry, Dr. Gihyun Sung from the Division of Advanced Materials Science at Pohang University of Science and Technology (POSTECH), Prof. Kyeng Min Park from Daegu Catholic University School of Medicine, Professor Hyun-Woo Rhee from the Department of Chemistry and Professor Jong-Seo Kim from the School of Biological Sciences at Seoul National University.
This work was supported by the National Research Foundation of Korea (NRF) and Institute for Basic Science (IBS).
Journal
Nature Communications
DOI
10.1038/s41467-024-46034-z
Article Title
OrthoID: profiling dynamic proteomes through time and space using mutually orthogonal chemical tools
Article Publication Date
29-Feb-2024




