Fukuoka, Japan—Researchers from Kyushu University have developed a hydrogen energy carrier to address some of the biggest hurdles in the path towards a sustainable hydrogen economy. As explained in a paper published in JACS Au, this novel compound can efficiently “store electrons” from hydrogen in a solid state to use in chemical reactions later.
Credit: Kyushu University/Seiji Ogo
Fukuoka, Japan—Researchers from Kyushu University have developed a hydrogen energy carrier to address some of the biggest hurdles in the path towards a sustainable hydrogen economy. As explained in a paper published in JACS Au, this novel compound can efficiently “store electrons” from hydrogen in a solid state to use in chemical reactions later.
Hydrogen is a promising source of clean energy with a lot of untapped potential applications in industry and everyday life. Unlike conventional fuels, hydrogen can be used to generate electricity without producing greenhouse gases. It can also be used in various chemical reactions such as hydrogenation, that is, as a source of hydride ion or hydrogen atom electrons. However, storing and transporting hydrogen in either gaseous or liquid states is extremely challenging, requiring expensive equipment and cooling systems.
Professor Seiji Ogo from Kyushu University’s WPI-International Institute for Carbon-Neutral Energy (WPI-I2CNER) has been developing innovative solutions to these problems. In their most recent study, Ogo and his colleague from Kindai University took inspiration from nature to develop an iridium-based compound with peculiar and remarkably useful properties.
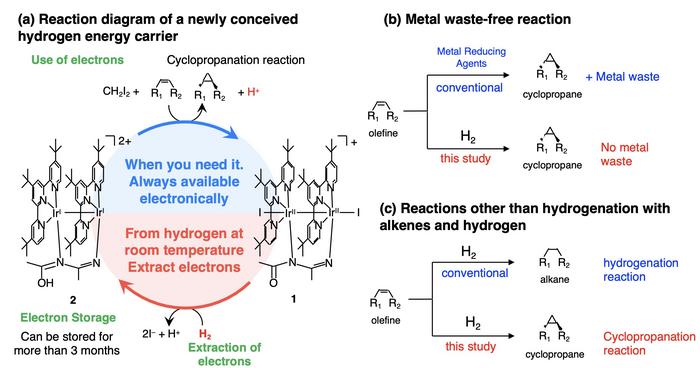
“We have been actively exploring hydrogen energy carriers that can be easily synthesized and used as-is. These compounds are based on the hydrogenase enzyme found in nature, which can catalyze hydrogen into protons and electrons at room temperature,” explains Ogo. “A core idea of our approach that led to a breakthrough was to view hydrogen not as a source of negatively charged hydride ion or hydrogen atom, but as electrons.”
After carefully examining many combinations of metal ions and organic ligands, the research team crafted an iridium-based compound which, when exposed to hydrogen incorporates it into the metal center after losing an iodide ion. In this way, the proposed compound can effectively extract and store electrons from hydrogen.
These changes are readily reversible under the right conditions, and the stored electrons can be easily extracted and used in chemical reactions to synthesize valuable molecules. In this study, the researchers focused on using the electrons stored in the compound to catalyze cyclopropanation reactions.
Cyclopropanes are molecules with a three-membered carbon ring structure and represent important structural units in various pharmaceutical drugs and organic compounds. However, conventional cyclopropanations have produced large amounts of waste metals as byproducts. The proposed hydrogen energy carrier circumvents this issue entirely.
“The cyclopropanation reactions performed in our study use hydrogen rather than metals as the reductant and thus produce no metal waste. This is a major advantage of the proposed compound over established techniques,” remarks Ogo.
Notably, this study also marks the first time that a reaction between hydrogen and alkenes—hydrocarbons containing a carbon double bond—produces cyclopropanes rather than the much simpler alkanes.
After extensive testing, the team found that the proposed energy carrier can capture electrons from hydrogen and store them for over three months in solid state at room temperature.
In future work, Ogo and colleagues plan to focus on developing a similar energy carrier using iron-group elements, which are cheaper and more abundant than iridium. By promoting industry-academia collaborations, their next efforts will aim to develop scalable solutions for practical problems surrounding upcoming hydrogen economies.
“We sincerely believe that the present achievement will contribute to the realization of a carbon-neutral society,” concludes Ogo.
###
For more information about this research, see “Cyclopropanation using Electrons Derived from Hydrogen—Reaction of Alkenes and Hydrogen without Hydrogenation”, Seiji Ogo, Takeshi Yatabe, Keishi Miyazawa, Yunosuke Hashimoto, Chiaki Takahashi, Hidetaka Nakai, and Yoshihito Shiota, JACS Au, DOI: https://doi.org/10.1021/jacsau.4c00098
About Kyushu University
Kyushu University is one of Japan’s leading research-oriented institutes of higher education since its founding in 1911. Home to around 19,000 students and 8,000 faculty and staff, Kyushu U’s world-class research centers cover a wide range of study areas and research fields, from the humanities and arts to engineering and medical sciences. Its multiple campuses—including one of the largest in Japan—are located around Fukuoka City, a coastal metropolis on the southwestern Japanese island of Kyushu that is frequently ranked among the world’s most livable cities and historically known as Japan’s gateway to Asia. Through its Vision 2030, Kyushu U will ‘Drive Social Change with Integrative Knowledge.’ Its synergistic application of knowledge will encompass all of academia and solve issues in society while innovating new systems for a better future.
About International Institute for Carbon-Neutral Energy Research (WPI-I²CNER) Kyushu University
Contribute to the creation of a sustainable and environmentally friendly society I²CNER’s mission is to contribute to the creation of a sustainable and environmentally-friendly society by advancing low-carbon emission and cost-effective energy systems, and improvement of energy efficiency. Through its mission-driven basic research, I²CNER has been developing and conducting research on scientific technologies to drastically reduce CO2 emissions.
https://i2cner.kyushu-u.ac.jp/en/
About the World Premier International Research Center Initiative (WPI)
The WPI program was launched in 2007 by Japan’s Ministry of Education, Culture, Sports, Science and Technology (MEXT) to foster globally visible research centers boasting the highest standards and outstanding research environments. Numbering more than a dozen and operating at institutions throughout the country, these centers are given a high degree of autonomy, allowing them to engage in innovative modes of management and research. The program is administered by the Japan Society for the Promotion of Science (JSPS).
See the latest research news from the centers at the WPI News Portal:
https://www.eurekalert.org/newsportal/WPI/home
Main WPI program site
https://www.jsps.go.jp/english/e-toplevel/
Journal
JACS Au
DOI
10.1021/jacsau.4c00098
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Cyclopropanation using Electrons Derived from Hydrogen—Reaction of Alkenes and Hydrogen without Hydrogenation
Article Publication Date
25-Mar-2024



