Mammalian mitochondrial electron transport chain complexes are the most important and complicated protein machinery in mitochondria. Although this system has been studied for more than a century, its composition and molecular mechanism are still largely unknown. Here, Yang’s group report the high-resolution cryo-electron microscopy (cryo-EM) structures of porcine respiratory chain megacomplex-I2III2IV2 (MCI2III2IV2) in five different conformations, including State 1, State 2, Mid 1, Mid 2, and Mid 3. High-resolution cryo-EM imaging, combined with super-resolution gated stimulated emission depletion microscopy (gSTED), strongly supports the formation of MCI2III2IV2 in live cells. Each MCI2III2IV2 structure contains 141 subunits (70 different kinds of peptides, 2.9 MDa) in total with 240 transmembrane helices. The mutual influence among CI, CIII, and CIV shown in the MCI2III2IV2 structure suggests this megacomplex could act as an integral unit in electron transfer and proton pumping. The conformational changes from different states suggest a plausible regulatory mechanism for the MCI2III2IV2 activation/deactivation process.
Credit: Maojun Yang ,School of Life Science, Tsinghua University
Mammalian mitochondrial electron transport chain complexes are the most important and complicated protein machinery in mitochondria. Although this system has been studied for more than a century, its composition and molecular mechanism are still largely unknown. Here, Yang’s group report the high-resolution cryo-electron microscopy (cryo-EM) structures of porcine respiratory chain megacomplex-I2III2IV2 (MCI2III2IV2) in five different conformations, including State 1, State 2, Mid 1, Mid 2, and Mid 3. High-resolution cryo-EM imaging, combined with super-resolution gated stimulated emission depletion microscopy (gSTED), strongly supports the formation of MCI2III2IV2 in live cells. Each MCI2III2IV2 structure contains 141 subunits (70 different kinds of peptides, 2.9 MDa) in total with 240 transmembrane helices. The mutual influence among CI, CIII, and CIV shown in the MCI2III2IV2 structure suggests this megacomplex could act as an integral unit in electron transfer and proton pumping. The conformational changes from different states suggest a plausible regulatory mechanism for the MCI2III2IV2 activation/deactivation process.
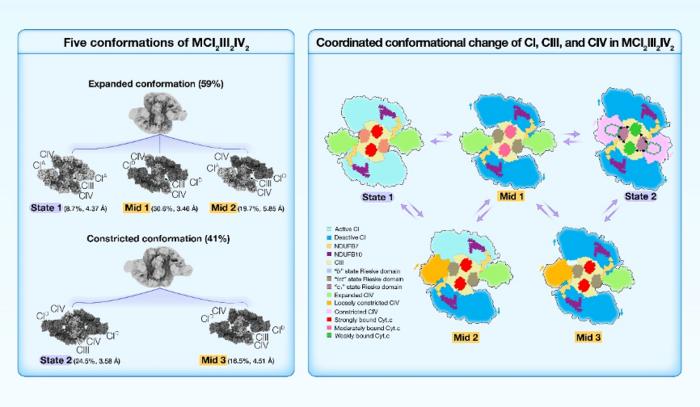
Yang’s Group solved the high-resolution structures of porcine MCI2III2IV2 in five conformations, State 1, State 2, Mid 1, Mid 2, and Mid 3, classified according to the conformation of CI, the Rieske domain of CIII, and the position of CIV. MCI2III2IV2 was classified into two conformations, expanded and constricted, based on the position of CIV. The two CIs found in constricted forms of MCI2III2IV2 were all in the deactive state. By contrast, the two CIs in the expanded conformations of MCI2III2IV2 could be found in either active or deactive states, suggesting that MCI2III2IV2 shifts from the active state (State 1) to the deactive state (State 2), via the intermediates (Mid 1, Mid 2, and Mid 3).
The dynamic range of the 2Fe-2S cluster in State 1 largely aligned with that reported in the “b” state; the dynamic range in Mid 1 largely coincided with that in the “int” state; and the dynamic range in State 2 overlapped between the “int” and “c1” states. So, when MCI2III2IV2 shifts from State 1 to State 2, the Rieske domain of CIII changes from the “b” state to the “c1” state.
Moreover, in both State 1 and Mid 1, the distances between the Cyt.c binding site of CIV and the two Cyt.c binding sites of CIII are around 95 Å and 115 Å, respectively, while in State 2, these distances are about 73 Å and 78 Å, respectively. This finding suggested that Cyt.c could potentially diffuse more readily over the markedly shorter distances between Cyt.c binding sites in State 2. In addition, we noted that the density of Cyt.c gradually scatters from State 1 to Mid 1, and to State 2, indicating that Cyt.c is more tightly bound to CIII in State 1, while more loosely bound in State 2.
As MCI2III2IV2 shifts from State 1 to State 2, two points of interaction between CI and CIII on the IMS side undergo significant conformational changes. In particular, a long helix (C80-G126) of NDUFB7 extends from the distal end of the CI membrane arm to contact the Rieske domain of CIII monomer A, and the C-terminus of NDUFB10 protrudes from the middle region of the CI membrane arm to contact the N-terminal region of UQCRH of CIII monomer B. In State 1, the closest distances between these two interaction sites are ~15.3 Å and ~11.9 Å, respectively, while in State 2, these distances are reduced to ~6.0 Å and ~7.9 Å, respectively. The Rieske domain is critical for electron transfer within CIII, and the N-terminal region of UQCRH is adjacent to the Cyt.c binding site of CIII. Consequently, NDUFB7A123 and NDUFB10A176 of CI may function as two anchor points to induce conformational changes in the CIII Rieske domain and Cyt.c binding site.
See the article:
Structural basis for the regulatory mechanism of mammalian mitochondrial respiratory chain megacomplex-I2III2IV2
Doi: 10.1016/j.hlife.2024.03.003
Journal
hLife
DOI
10.1016/j.hlife.2024.03.003
Article Title
Structural basis for the regulatory mechanism of mammalian mitochondrial respiratory chain megacomplex-I2III2IV2
Article Publication Date
15-Mar-2024