“These results suggest strong potential for clinical use of the assay in ctDNA monitoring of solid tumor cancers.”
Credit: 2024 Northcott et al.
“These results suggest strong potential for clinical use of the assay in ctDNA monitoring of solid tumor cancers.”
BUFFALO, NY- March 20, 2024 – A new research paper was published in Oncotarget’s Volume 15 on March 14, 2024, entitled, “Analytical validation of NeXT Personal®, an ultra-sensitive personalized circulating tumor DNA assay.”
In this new study, researchers Josette Northcott, Gabor Bartha, Jason Harris, Conan Li, Fabio C.P. Navarro, Rachel Marty Pyke, Manqing Hong, Qi Zhang, Shuyuan Ma, Tina X. Chen, Janet Lai, Nitin Udar, Juan-Sebastian Saldivar, Erin Ayash, Joshua Anderson, Jiang Li, Tiange Cui, Tu Le, Ruthie Chow, Randy Jerel Velasco, Chris Mallo, Rose Santiago, Robert C. Bruce, Laurie J. Goodman, Yi Chen, Dan Norton, Richard O. Chen, and John M. Lyle from Personalis, Inc. describe the analytical validation of NeXT Personal®, an ultra-sensitive, tumor-informed circulating tumor DNA (ctDNA) assay for detecting residual disease, monitoring therapy response, and detecting recurrence in patients diagnosed with solid tumor cancers.
“NeXT Personal uses whole genome sequencing of tumor and matched normal samples combined with advanced analytics to accurately identify up to ~1,800 somatic variants specific to the patient’s tumor.”
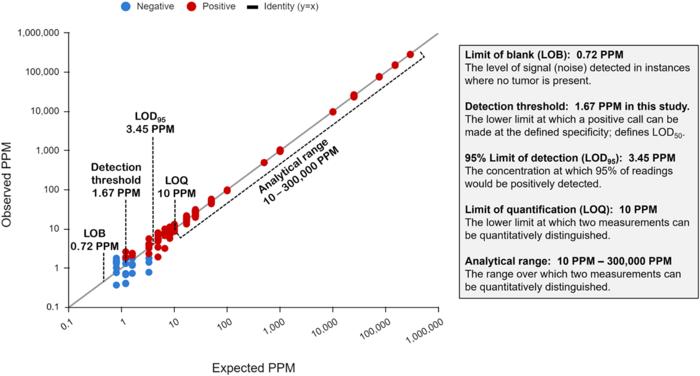
A personalized panel is created, targeting these variants and then used to sequence cell-free DNA extracted from patient plasma samples for ultra-sensitive detection of ctDNA. The NeXT Personal analytical validation is based on panels designed from tumor and matched normal samples from two cell lines, and from 123 patients across nine cancer types. Analytical measurements demonstrated a detection threshold of 1.67 parts per million (PPM) with a limit of detection at 95% (LOD95) of 3.45 PPM. NeXT Personal showed linearity over a range of 0.8 to 300,000 PPM (Pearson correlation coefficient = 0.9998). Precision varied from a coefficient of variation of 12.8% to 3.6% over a range of 25 to 25,000 PPM. The assay targets 99.9% specificity, with this validation study measuring 100% specificity and in silico methods giving a confidence interval of 99.92 to 100%.
“In summary, this study demonstrates NeXT Personal as an ultra-sensitive, highly quantitative and robust ctDNA assay that can be used to detect residual disease, monitor treatment response, and detect recurrence in patients.”
Continue reading: DOI: https://doi.org/10.18632/oncotarget.28565
Correspondence to: John M. Lyle
Email: [email protected]
Keywords: circulating tumor DNA, whole genome sequencing, molecular residual disease, tumor-informed assay, analytical validation
Click here to sign up for free Altmetric alerts about this article.
About Oncotarget: Oncotarget (a primarily oncology-focused, peer-reviewed, open access journal) aims to maximize research impact through insightful peer-review; eliminate borders between specialties by linking different fields of oncology, cancer research and biomedical sciences; and foster application of basic and clinical science.
Oncotarget is indexed and archived by PubMed/Medline, PubMed Central, Scopus, EMBASE, META (Chan Zuckerberg Initiative) (2018-2022), and Dimensions (Digital Science).
To learn more about Oncotarget, visit Oncotarget.com and connect with us on social media:
- X, formerly Twitter
- YouTube
- Spotify, and available wherever you listen to podcasts
Click here to subscribe to Oncotarget publication updates.
For media inquiries, please contact [email protected].
Oncotarget Journal Office
6666 East Quaker Street., Suite 1A
Orchard Park, NY 14127
Phone: 1-800-922-0957 (option 2)
###
Journal
Oncotarget
DOI
10.18632/oncotarget.28565
Method of Research
Experimental study
Subject of Research
People
Article Title
Analytical validation of NeXT Personal®, an ultra-sensitive personalized circulating tumor DNA assay
Article Publication Date
14-Mar-2024




