Is there a way to stick hard and soft materials together without any tape, glue or epoxy? A new study published in ACS Central Science shows that applying a small voltage to certain objects forms chemical bonds that securely link the objects together. Reversing the direction of electron flow easily separates the two materials. This electroadhesion effect could help create biohybrid robots, improve biomedical implants and enable new battery technologies.
Credit: Adapted from ACS Central Science 2024, DOI:10.1021/acscentsci.3c01593
Is there a way to stick hard and soft materials together without any tape, glue or epoxy? A new study published in ACS Central Science shows that applying a small voltage to certain objects forms chemical bonds that securely link the objects together. Reversing the direction of electron flow easily separates the two materials. This electroadhesion effect could help create biohybrid robots, improve biomedical implants and enable new battery technologies.
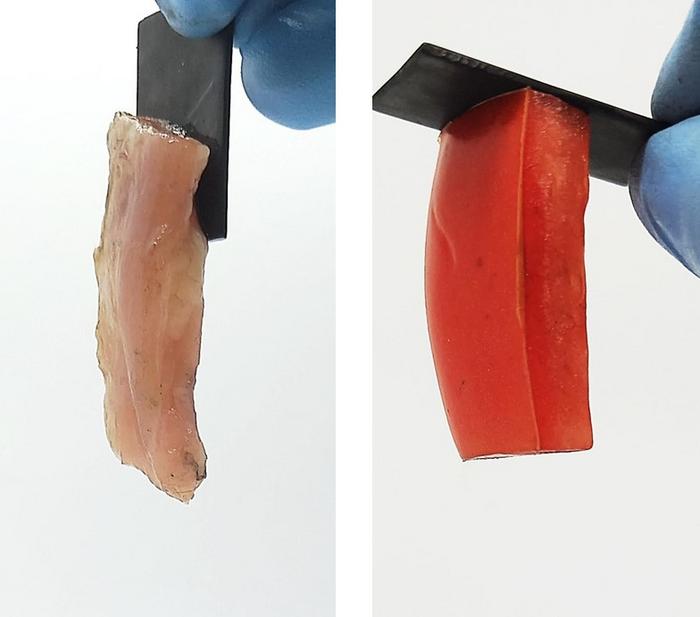
When an adhesive is used to attach two things, it binds the surfaces either through mechanical or electrostatic forces. But sometimes those attractions or bonds are difficult, if not impossible, to undo. As an alternative, reversible adhesion methods are being explored, including electroadhesion (EA). Though the term is used to describe a few different phenomena, one definition involves running an electric current through two materials causing them to stick together, thanks to attractions or chemical bonds. Previously, Srinivasa Raghavan and colleagues demonstrated that EA can hold soft, oppositely charged materials together, and even be used to build simple structures. This time, they wanted to see if EA could reversibly bind a hard material, such as graphite, to a soft material, such as animal tissue.
The team first tested EA using two graphite electrodes and an acrylamide gel. A small voltage (5 volts) was applied for a few minutes, causing the gel to permanently adhere to the positively charged electrode. The resulting chemical bond was so strong that, when one of the researchers tried to wrench the two pieces apart, the gel tore before it disconnected from the electrode. Notably, when the current’s direction was reversed, the graphite and gel easily separated — and the gel instead adhered to the other electrode, which was now positively charged. Similar tests were run on a variety of materials — metals, various gel compositions, animal tissues, fruits and veggies — to determine the phenomenon’s ubiquity.
For EA to occur, the authors found that the hard material needs to conduct electrons, and the soft material needs to contain salt ions They hypothesize that the adhesion arises from chemical bonds that form between the surfaces after an exchange of electrons. This may explain why some metals that hold onto their electrons strongly, including titanium, and some fruits that contain more sugar than salts, including grapes, failed to adhere in some situations. A final experiment showed that EA can occur completely underwater, revealing an even wider range of possible applications. The team says that this work could help create new batteries, enable biohybrid robotics, enhance biomedical implants and much more.
The authors do not acknowledge a funding source for this work.
The paper’s abstract will be available on Mar. 13 at 8 a.m. Eastern time here: http://pubs.acs.org/doi/abs/10.1021/acscentsci.3c01593
For more of the latest research news, register for our upcoming meeting, ACS Spring 2024. Journalists and public information officers are encouraged to apply for complimentary press registration by completing this form.
###
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people. The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News. ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a leader in scientific information solutions, its CAS division partners with global innovators to accelerate breakthroughs by curating, connecting and analyzing the world’s scientific knowledge. ACS’ main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive news releases from the American Chemical Society, contact [email protected].
Note: ACS does not conduct research, but publishes and publicizes peer-reviewed scientific studies.
Follow us: X, formerly Twitter | Facebook | LinkedIn | Instagram
Journal
ACS Central Science
DOI
10.1021/acscentsci.3c01593
Article Title
Reversibly Sticking Metals and Graphite to Hydrogels and Tissues
Article Publication Date
13-Mar-2024




