A groundbreaking discovery in the field of catalysis has emerged from the laboratories of Professor Jaeheung Cho and his team in the Department of Chemistry at UNIST. Their pioneering work has led to the development of a copper(II)–alkylperoxo complex that promises to revolutionize the realms of synthetic chemistry and industrial applications.
Credit: UNIST
A groundbreaking discovery in the field of catalysis has emerged from the laboratories of Professor Jaeheung Cho and his team in the Department of Chemistry at UNIST. Their pioneering work has led to the development of a copper(II)–alkylperoxo complex that promises to revolutionize the realms of synthetic chemistry and industrial applications.
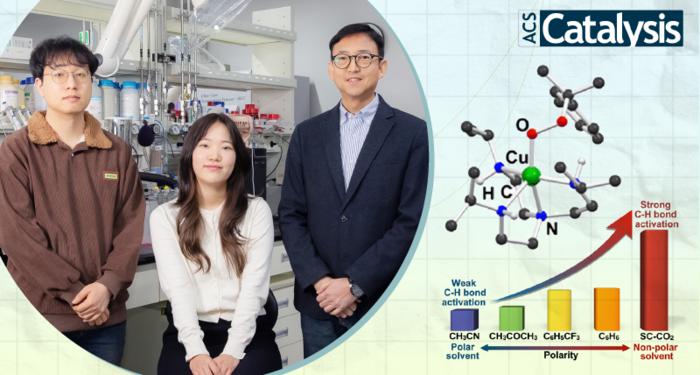
The key to this innovative catalyst lies in its remarkable ability to decompose strong carbon-hydrogen bonds (C−H bonds) through harnessing specific solvents. By precisely manipulating the solvent environment, the researchers have uncovered the exceptional reactivity of their copper(II)–alkylperoxo complex.
Through a meticulously designed series of experiments, the research team successfully synthesized the copper(II)–alkylperoxo complex and subjected it to supercritical carbon dioxide (SC-CO2), a fluid state of carbon dioxide that exhibit both gas and liquid properties simultaneously. This novel approach resulted in the most reactive metal–alkylperoxo peroxide compound to date.
Professor Cho highlighted, “Our comprehensive analysis of oxidation reactions and advanced theoretical calculations have introduced a new era in oxidation catalysis utilizing copper(II)–alkylperoxo as a catalyst.”
Of particular significance is the oxidation of unactivated alkanes, like methane and ethane, traditionally known for their stability and energy-intensive oxidation processes. By tailoring the copper(II)–alkylperoxo complex composition, the researchers achieved selective oxidation of unactivated alkanes, a pivotal advancement in catalytic science. Moreover, the team’s exploration of various solvents confirmed the unprecedented ability of their catalyst to break down resilient C−H bonds.
Yuri Lee, the first author of the study, emphasized, “Our research signifies a milestone in reactivity manipulation through solvent engineering within copper(II)–alkylperoxo species.”
Professor Cho further underlined, “Our work not only showcases the exceptional oxidation capabilities of copper(II)–alkylperoxo species, but also elucidates their solvent-dependent reactivity, laying the foundation for cutting-edge metal catalysts in various scientific domains.”
The research team anticipates that this transformative research not only propels the boundaries of synthetic chemistry, but also holds immense promise for environmental and industrial applications, heralding a new era of catalytic excellence and sustainable technology. This research, with Professor Jaeheung Cho as the corresponding author, was published in the online version of ACS Catalysis on February 20, 2024. The study received funding from the National Research Foundation of Korea (NRF) and the Ministry of Science and ICT (MSIT), highlighting its importance in advancing eco-friendly technologies and catalytic innovation.
Journal Reference
Yuri Lee, Bohee Kim, Seonghan Kim, et al., “Influence of Solvents on Catalytic C–H Bond Oxidation by a Copper(II)–Alkylperoxo Complex,” ACS Catal., (2024)
Journal
ACS Catalysis
Article Title
Influence of Solvents on Catalytic C–H Bond Oxidation by a Copper(II)–Alkylperoxo Complex
Article Publication Date
20-Feb-2024




