Professor Jian HE, from the Department of Chemistry at The University of Hong Kong (HKU), has spearheaded a research endeavour aimed at revolutionising organic synthesis. His research team has successfully developed a novel heterogeneous copper photocatalyst that enables the efficient formation of cyclobutane rings, a crucial structural element in a vast array of bioactive molecules. Cyclobutane rings are prominently featured in pharmaceuticals, natural products, and various biologically active compounds. By enabling researchers to construct these rings easily and selectively, Professor He’s team has unlocked greater control over the synthesis of these vital molecules. The research findings are recently published online in the top scientific journal Nature Catalysis.
Credit: The University of Hong Kong
Professor Jian HE, from the Department of Chemistry at The University of Hong Kong (HKU), has spearheaded a research endeavour aimed at revolutionising organic synthesis. His research team has successfully developed a novel heterogeneous copper photocatalyst that enables the efficient formation of cyclobutane rings, a crucial structural element in a vast array of bioactive molecules. Cyclobutane rings are prominently featured in pharmaceuticals, natural products, and various biologically active compounds. By enabling researchers to construct these rings easily and selectively, Professor He’s team has unlocked greater control over the synthesis of these vital molecules. The research findings are recently published online in the top scientific journal Nature Catalysis.
In recent two decades, visible-light photocatalysts have been employed to drive such transformations through photochemical [2+2] cycloadditions; however, their substrate scope has been limited. Moreover, existing photocatalytic systems often rely on homogeneous precious metal catalysts, which pose challenges in catalyst recycling and hinder large-scale organic synthesis.
Addressing these limitations head-on, Professor He and his team have developed an innovative heterogeneous copper photocatalyst. This catalyst effectively facilitates energy-transfer processes for a range of intermolecular crossed [2+2] cycloadditions, including those previously inaccessible in conventional homogeneous photocatalysis. This new reaction system has excellent catalyst stability and recyclability, and it does not rely on precious metals, making it more economically and environmentally sustainable.
Professor He expressed great enthusiasm for the potential impact of this discovery, stating, ‘Our novel heterogeneous copper photocatalyst opens up new possibilities for synthesising bioactive molecules with enhanced efficiency and selectivity. By eliminating the reliance on precious metals and improving catalyst recyclability, we have addressed critical challenges in large-scale organic synthesis, paving the way for more sustainable and economically viable chemical production.’
Key findings
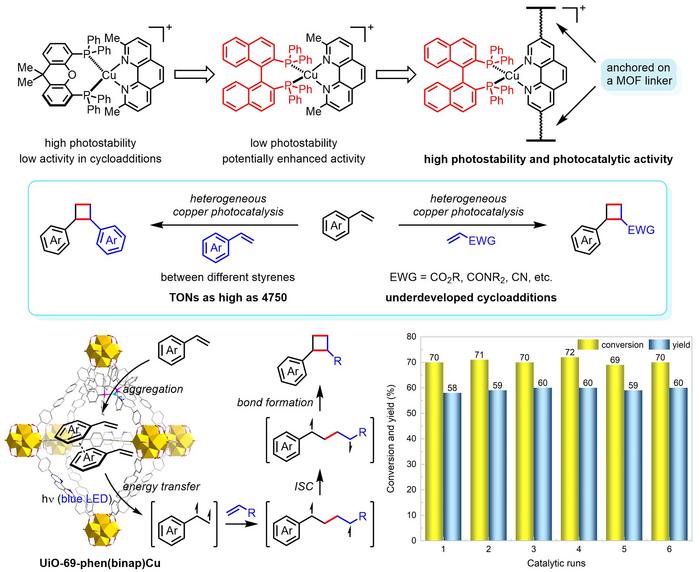
In this study, the team developed a special kind of copper photocatalyst that can help make cyclobutane molecules more efficiently. They successfully prepared a stable heterogeneous copper triplet photosensitiser by constructing binap-ligated heteroleptic copper(I) complexes in the pores of zirconium-based metal–organic frameworks (MOFs). Upon heterogenisation, reactive copper(I) species in photoexcited states show increased transition energies and lifetime, which is essential for boosting catalytic efficiency in energy-transfer-mediated [2+2] cycloadditions of styrenes with a variety of olefins, including electron-deficient alkenes. Unlike the corresponding homogeneous copper photocatalysts, the MOF-supported copper photocatalyst exhibits high stability and catalytic activity, and can be recycled multiple runs without catalyst decomposition. This work provides a general and interdisciplinary approach to designing highly reactive copper photocatalysts for a wide range of practical organic reactions, and it could help make the process of producing diverse bioactive compounds easier and more efficient in the future.
About Professor Jian He
Professor Jian He is an Assistant Professor in the Department of Chemistry and State Key Laboratory of Synthetic Chemistry at The University of Hong Kong. His independent research programme, at the interface between organic chemistry, inorganic chemistry, and materials science, is rooted in the design of novel framework- and cluster-based catalysts for the advancement of sustainable organic synthesis. He has received multiple awards, including The Alfred R. Bader Award for Student Innovation in 2014, International Precious Metals Institute Student Award in 2015, Reaxys PhD Prize Finalist in 2015, ACS Division of Organic Chemistry Travel Award in 2015, Chinese Government Award for Outstanding Self-Financed Students Abroad in 2015, IUPAC-Solvay Honorable Mention Prize in 2017, and Croucher Innovation Award in 2021.
About the Research Team
Dr Jun GUO and Mr Qi XIA from Professor HE’s group are the co-first authors. Other researchers, including Wing Yi TANG, Zekun LI, Xia WU, Li-Juan LIU, Wai-Pong TO, Hui-Xing SHU, Kam-Hung LOW, and Professor Philip C. Y. CHOW from HKU and Professor Tsz Woon Benedict LO from The Hong Kong Polytechnic University, contribute to this project.
Journal title: ‘Visible light-mediated intermolecular crossed [2+2] cycloadditions using a MOF-supported copper triplet photosensitizer’ (Nature Catalysis, 2024).
The journal paper can be accessed here: https://www.nature.com/articles/s41929-024-01112-9
Image and caption for download: https://www.scifac.hku.hk/press
For media enquiries, please contact Ms Casey To, External Relations Officer (tel: 39174948; email: [email protected] / Ms Cindy Chan, Assistant Director of Communications of HKU Faculty of Science (tel: 3917 5286; email: [email protected]).
Journal
Nature Catalysis
DOI
10.1038/s41929-024-01112-9
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Visible light-mediated intermolecular crossed [2+2] cycloadditions using a MOF-supported copper triplet photosensitizer
Article Publication Date
23-Feb-2024




