The mechanisms by which antidepressants and other emotion-focused medications work could be reconsidered due to an important new breakthrough in the understanding of how the gut communicates with the brain.
Credit: Flinders University
The mechanisms by which antidepressants and other emotion-focused medications work could be reconsidered due to an important new breakthrough in the understanding of how the gut communicates with the brain.
New research led by Flinders University has uncovered major developments in understanding how the gut communicates with the brain, which could have a profound impact on the make-up and use of medications such as antidepressants.
“The gut-brain axis consists of complex bidirectional neural communication pathway between the brain and the gut, which links emotional and cognitive centres of the brain,” says Professor Nick Spencer from the College of Medicine and Public Health.
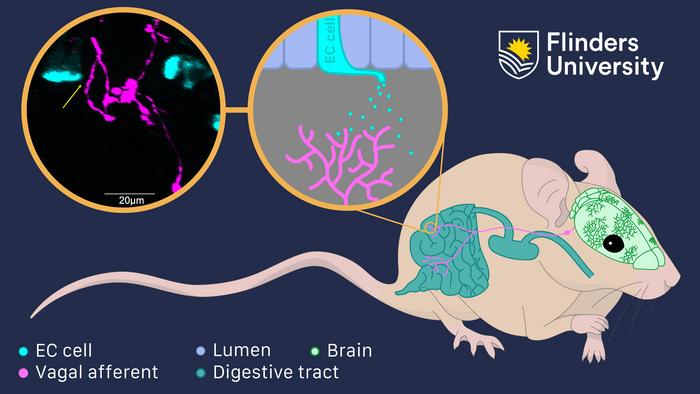
“As part of the gut-brain axis, vagal sensory nerves relay a variety of signals from the gut to the brain that play an important role in mental health and wellbeing.
“The mechanisms by which vagal sensory nerve endings in the gut wall are activated has been a major mystery but remains of great interest to medical science and potential treatments for mental health and wellbeing.”
Serotonin is a major hormone and neurotransmitter in the body and has been shown to play a major role in a range of bodily functions, including mental health and depression.
“The vast majority of serotonin is made in the gut in specialised cells, called enteroendocrine cells (EECs), within the gut wall but we still don’t understand how serotonin released from EECs activates the sensory nerve endings of the vagus nerve,” says Professor Spencer.
“It had once been proposed that EECs make physical synaptic connections with the sensory nerve endings of the vagus and use fast neurotransmitters to activate vagal sensory endings.
“However, the results of our new research uncover that any substances (including serotonin) released from EEC cells must communicate via a process of diffusion to the sensory nerve endings of the vagus nerve, that lie in colon (large intestine).
“We found that the distances between individual EECs that contain serotonin and vagal afferent nerve endings were too far apart to occur via a mechanism that involved synaptic communication and fast neurotransmission, as was previously thought.
“This is a major discovery for our understanding of gut-brain communication which has profound implications for drug developments, treatments of anxiety and depression and other digestive problems such as irritable bowel symptom (IBS), all of which involve serotonin in some way.
“It opens a whole new way of thinking and scientific enquiry for future drug development and investigation for control of the gut-brain axis and potential treatments for mental health and wellbeing, for instance the use of selective serotonin reuptake inhibitors (SSRIs), a widely used type of antidepressant,” he says.
“The majority of serotonin in the body, around 95%, originates in the gut, so there is great interest in how such large quantities of serotonin released from EEC cells act on the vagal sensory nerve endings in the gut wall.
“Understanding this mechanism can provide major new clues as to how serotonin not only communicates along the gut-brain axis, but how this serotonin may be involved in the control of health and wellbeing,” he says.
“Up until now, how different substances (like serotonin) released from EECs activated vagal nerve endings in the gut has been unresolved. Recent literature suggests that that this communication occurred through physical connections known as synapses, and that EEC cells form very close junctions with vagal sensory endings.
“Our findings show that any substances released from EECs must act via diffusion onto vagal sensory nerve endings in the colon, which then relay sensory information to the brain,” says Professor Spencer.
Synaptic transmission is the process by which neurotransmitters communicate with a target cell or cells, for example another neuron(s) or muscle cell(s), via a specialised structure known as the synapse. This involves the neurotransmitter molecules crossing a very short distance to their target cell(s).
Diffusion is the net movement of molecules from one region to another that can occur over any distance.
The researchers used an intricate method of anterograde neuronal tracing from the sensory nerve cell bodies of the vagus nerve, which lie just outside the brain, but send their long nerve projections (axons) all the way down to the small intestine and proximal part of the colon.
“The mean distances between vagal nerve endings and the nearest serotonin containing EECs were hundreds of times greater than known distances that underlie synaptic transmission in vertebrates. This rules out any possible mechanism of fast synaptic transmission,” says Professor Spencer.
“The absence of any close physical contacts between serotonin-containing EECs and vagal nerve endings in our studies leads to the inescapable conclusion that the mechanism by which serotonin activates the sensory nerve endings of the vagus nerve is by diffusion.
“What the findings confirm is that substances released from EECs must communicate via diffusion to activate vagal sensory nerve endings.
“Our understanding of how the gut communicates with the brain, via sensory nerves has been substantially improved based on the findings of this study, and we look forward to exploring this topic further,” he adds.
The paper, Identification of vagal afferents nerve endings in the mouse colon and their spatial relationship with enterochromaffin cells by Nicholas J. Spencer, Melinda Kyloh, Lee Travis and Tim Hibberd was published in Cell and Tissue Research. DOI : 10.1007/s00441-024-03879-6
Journal
Cell and Tissue Research
DOI
10.1007/s00441-024-03879-6
Method of Research
Experimental study
Subject of Research
Animals
Article Title
Identification of vagal afferents nerve endings in the mouse colon and their spatial relationship with enterochromaffin cells
Article Publication Date
22-Feb-2024
COI Statement
The authors declare no competing interests.




