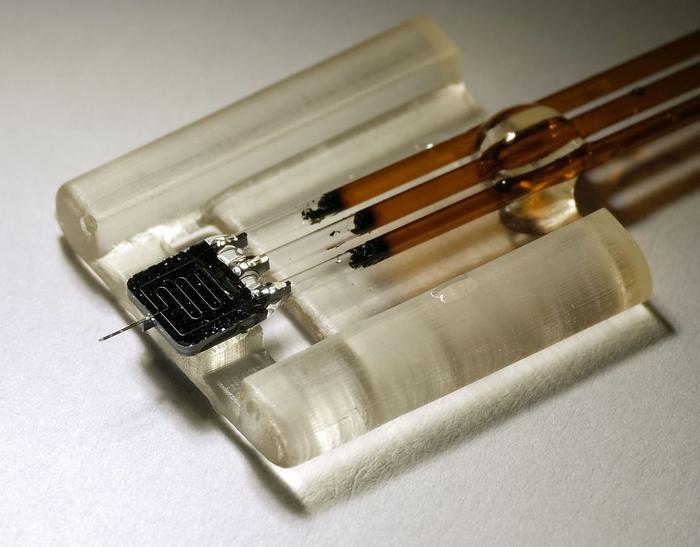
Longstanding challenges in biomedical research such as monitoring brain chemistry and tracking the spread of drugs through the body require much smaller and more precise sensors. A new nanoscale sensor that can monitor areas 1,000 times smaller than current technology and can track subtle changes in the chemical content of biological tissue with sub-second resolution, greatly outperforming standard technologies.
Credit: The Grainger College of Engineering at University of Illinois Urbana-Champaign
Longstanding challenges in biomedical research such as monitoring brain chemistry and tracking the spread of drugs through the body require much smaller and more precise sensors. A new nanoscale sensor that can monitor areas 1,000 times smaller than current technology and can track subtle changes in the chemical content of biological tissue with sub-second resolution, greatly outperforming standard technologies.
The device, developed by researchers at the University of Illinois Urbana-Champaign, is silicon-based and takes advantage of techniques developed for microelectronics manufacturing. The small device size enables it to collect chemical content with close to 100% efficiency from
highly localized regions of tissue in a fraction of a second. The capabilities of this new nanodialysis device are reported in the journal ACS Nano.
“With our nanodialysis device, we take an established technique and push it into a new extreme, making biomedical research problems that were impossible before quite feasible now,” said Yurii Vlasov, a U. of I. electrical & computer engineering professor and a co-lead of the study.
“Moreover, since our devices are made on silicon using microelectronics fabrication techniques, they can be manufactured and deployed on large scales.”
From micro- to nanodialysis
Nanodialysis is based on a technique called microdialysis in which a probe with a thin membrane is inserted into biological tissue. Chemicals pass through the membrane into a fluid that is pumped away for analysis. The ability to directly sample from tissue has made a major impact in fields like neuroscience, pharmacology and dermatology.
Traditional microdialysis has limitations, though. The probes sample from a few square
millimeters, so they can only measure the average composition over relatively large regions in the tissue. The large size also results in some degree of tissue damage when the probe is inserted, potentially skewing the analysis results. Finally, the fluid pumped through the probe flows at a comparatively high rate, impacting the efficiency and accuracy with which chemical concentrations can be read.
“Many problems with traditional microdialysis can be solved by using a much smaller device,” Vlasov said. “Going smaller with nanodialysis means more precision, less damage from the tissue placement, chemically mapping the tissue with higher spatial resolution, and a much faster readout time allowing a more detailed picture of the changes in tissue chemistry.”
Slow and steady
The most important feature of nanodialysis is the ultra-slow flow rate of the fluid pumped
through the probe. By making the flow rate 1,000 times slower than traditional microdialysis, the device captures the chemical composition of the tissue collected from an area 1,000 times smaller than traditional techniques while maintaining 100% efficiency.
“By drastically decreasing the flow rate, it allows the chemicals diffusing into the probe to match the concentrations outside in the tissue,” Vlasov explained. “Imagine you’re adding dye to a pipe with flowing water. If the flow is too fast, the dye gets diluted to concentrations that are difficult to detect. To avoid dilution, you need to turn the water almost all the way down.”
Silicon fabrication and production
Standard microdialysis devices are constructed using glass probes and polymer membranes, making them a challenge to miniaturize. To build devices suitable for nanodialysis, the researchers used techniques developed for electronic chip manufacturing to create a device based on silicon.
“In addition to enabling us to go smaller, silicon technology makes the devices cheaper,” Vlasov said. “By putting in the time and effort to develop a fabrication process for building our nanodevices on silicon, it is now very straightforward to manufacture them at industrial scales at an incredibly low cost.”
***
Rashid Bashir, a U. of I. bioengineering professor and the dean of The Grainger College of
Engineering, co-led the project.
These results are published in the ACS Nano article “Highly localized chemical sampling at sub-second temporal resolution enabled with a silicon nanodialysis platform at exceedingly slow flows.” DOI: 10.1021/acsnano.3c09776
Support was provided by the National Institutes of Health’s Brain Research Through Advancing Innovative Neurotechnologies Initiative.
Journal
ACS Nano
DOI
10.1021/acsnano.3c09776
Article Title
Highly Localized Chemical Sampling at Subsecond Temporal Resolution Enabled with a Silicon Nanodialysis Platform at Nanoliter per Minute Flows
Article Publication Date
20-Feb-2024




