Osaka, Japan – The chemical industry commonly uses rare, expensive metals to produce pharmaceuticals and other essential substances. Replacing these metals whenever possible with more-abundant, cheaper substitutes would benefit environmental sustainability, lower costs, and minimize the risk of supply chain disruptions.
Credit: 2024 Yamaguchi et al., Nickel Carbide Nanoparticle Catalyst for Selective Hydrogenation of Nitriles to Primary Amines, Chemistry – A European Journal
Osaka, Japan – The chemical industry commonly uses rare, expensive metals to produce pharmaceuticals and other essential substances. Replacing these metals whenever possible with more-abundant, cheaper substitutes would benefit environmental sustainability, lower costs, and minimize the risk of supply chain disruptions.
Now, in a study recently published in Chemistry – A European Journal, researchers from Osaka University and collaborating partners have met this need in their work on an industrially useful chemical transformation. The simple, gentle reaction conditions reported here might inspire researchers who are working to reduce use of expensive metals for as many chemical reactions as possible.
So-called noble metals are especially versatile materials. For example, palladium is a metal of choice for catalyzing a chemical transformation – converting nitriles into primary amines – that is a common step in nylon and plastics production. However, such metals are rare and costly. Substitutes based on common metals such as nickel could be cheaper catalysts. Unfortunately, many cheap metals require challenging experimental conditions, such as high pressures and temperatures, for the previously mentioned chemical transformation. Determining whether nickel carbide has the same limitations – and if not, evaluating the scope of the chemical transformations that are possible with this catalyst – was the goal of the research team’s study.
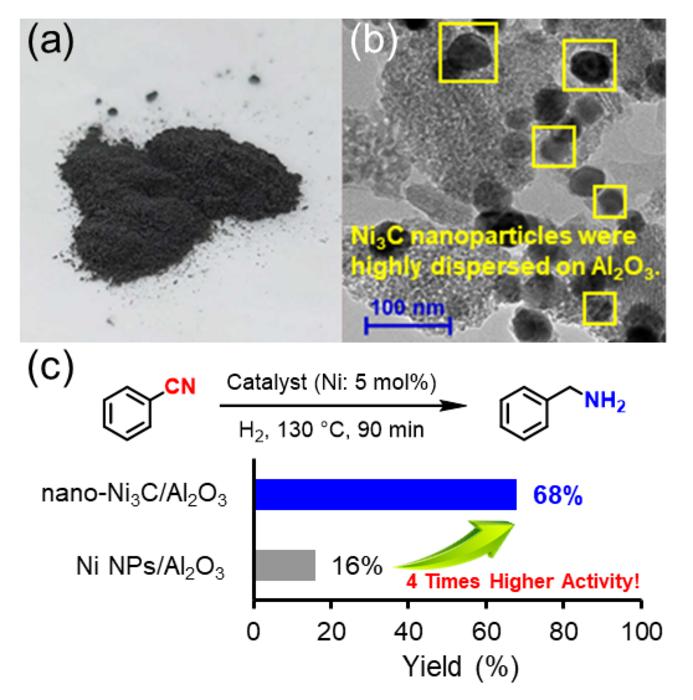
“In our work, we thoroughly study the reaction chemistry that underlies a novel nickel carbide nanoparticle heterogeneous catalyst for selective hydrogenation of nitriles into primary amines,” explains Sho Yamaguchi, lead author of the study. “The substrate scope is broad – many types of heteroaromatic and aliphatic nitriles can undergo this transformation.”
There are several advantages of the researchers’ catalyst. One, despite the mild required reaction conditions – 1 atmosphere pressure of hydrogen and a relatively low temperature of approximately 150°C – the catalyst still exhibited 4-times the activity of simple nickel nanoparticles. Two, the catalyst was reusable: at least 3-times. Three, the reaction yields were high: up to 99%.
“We’re excited because our research will help minimize the use of expensive metals for and simplifies the experimental setup of a common class of chemical syntheses,” says Tomoo Mizugaki, senior author. “Furthermore, our theoretical calculations provide insights that will help us optimize the catalyst for additional applications.”
This work is an important step forward in increasing the sustainability of a class of chemical reactions that are required for synthesizing pharmaceuticals and many other everyday products. Because the nickel catalyst is much cheaper than a noble metal, and the required experimental procedures are simple, feasible applications to further chemical transformations should be straightforward.
###
The article, “Nickel carbide nanoparticle catalyst for selective hydrogenation of nitriles to primary amines,” was published in Chemistry – A European Journal at DOI: https://doi.org/10.1002/chem.202303573
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and is now one of Japan’s leading comprehensive universities with a broad disciplinary spectrum. This strength is coupled with a singular drive for innovation that extends throughout the scientific process, from fundamental research to the creation of applied technology with positive economic impacts. Its commitment to innovation has been recognized in Japan and around the world, being named Japan’s most innovative university in 2015 (Reuters 2015 Top 100) and one of the most innovative institutions in the world in 2017 (Innovative Universities and the Nature Index Innovation 2017). Now, Osaka University is leveraging its role as a Designated National University Corporation selected by the Ministry of Education, Culture, Sports, Science and Technology to contribute to innovation for human welfare, sustainable development of society, and social transformation.
Website: https://resou.osaka-u.ac.jp/en
Journal
Chemistry – A European Journal
DOI
10.1002/chem.202303573
Method of Research
Experimental study
Subject of Research
Lab-produced tissue samples
Article Title
Nickel Carbide Nanoparticle Catalyst for Selective Hydrogenation of Nitriles to Primary Amines
Article Publication Date
5-Jan-2024




