Clathrate hydrates are s
Credit: Youngjune Park from Gwangju Institute of Science and Technology, Korea
Clathrate hydrates are s
However, using them in real-world applications is still a significant challenge as they have limited gas storage capacities and slow formation rates that require high pressure and low temperature conditions. In a new study led by Professor Youngjune Park from the Gwangju Institute of Science and Technology in Korea, a team of researchers has comprehensively reviewed recent research findings, including their own, that address these myriad challenges associated with clathrate hydrates. According to Prof. Park: “By exploring the untapped potential of clathrate hydrates, we have effectively mitigated these limitations and provided efficient hydrogen (H2) storage solutions through the introduction of hydrogen-natural gas blends into clathrate hydrates.” Their study was made available online on 7 November 2023 and published in the journal Accounts of Chemical Research on 21 November 2023.
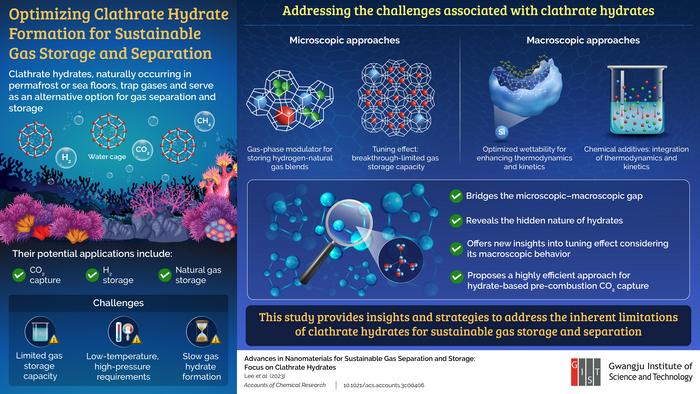
The team presents an account of macroscopic and microscopic approaches to bridge the gap between them. The microscopic approach includes techniques like using ‘gas-phase modulators’ to synthesize hydrates which enable concurrent storage of light hydrocarbons and hydrogen and prevent chemical waste generation. Tuning effects, which aid in maximizing gas storage capacity and promote thermodynamic stability, are also discussed. From a macroscopic viewpoint, the study included interfacial interactions with porous substances and how they accelerate kinetics to enhance hydrate formation. Additionally, rapid formation by chemical additives was also summarized.
This study thus helps to advance the untapped potential of gas hydrates by bridging macroscopic and microscopic properties, and the exploration of their hidden nature opens various potential applications. These encompass efficient techniques for pre- and post-combustion CO2 capture using hydrates, demonstrating practical and economic viability. Subsequently, this can reduce carbon dioxide emissions from power plants and thereby play a role in mitigating climate change in the long run and making carbon capture more affordable.
These insights will also help to increase the storage and transportation capacity of hydrogen and natural gas, which is crucial for industries involved in energy applications using these gases. “This research has significant long-term potential for the gas storage and separation industry, particularly in achieving carbon neutrality. Our findings offer crucial insights for developing clathrate hydrate-based technologies for hydrogen storage and carbon dioxide separation, essential for a future low-carbon society,” emphasizes Prof. Park.
Other sustainable applications include gas separation processes, as well as desalination processes, thereby providing an avenue for the treatment of water and other substances in industries requiring large-scale processing. Clathrates could also contribute to the storage and transportation of gases produced in renewable energy processes, such as biogas or syngas from biomass or waste.
In effect, this study underscores the essential link between nanostructures and their macroscopic characteristics, highlighting the importance of comprehending their interaction for economic viability. These reveal the concealed essence of gas hydrates and can help shape new approaches to tackle challenges and establish the foundation for tangible practical applications!
***
Reference
Title of original paper: Advances in Nanomaterials for Sustainable Gas Separation and Storage: Focus on Clathrate Hydrates
Journal: Accounts of Chemical Research
DOI: https://doi.org/10.1021/acs.accounts.3c00406
About the institute
The Gwangju Institute of Science and Technology (GIST) is a renowned research-oriented university in Gwangju, South Korea, with a primary focus on science and technology disciplines. Established in 1993, GIST has evolved into one of South Korea’s leading institutions for higher education and cutting-edge research. GIST was ranked 96th in the world in the Engineering & Technology category of the Times Higher Education World University Rankings for 2014–2015, and is a member of a consortium of research-oriented universities, which includes GIST-KAIST-UNIST-UST-POSTECH-DGIST.
About the author
Youngjune Park is a Professor at the School of Earth Sciences and Environmental Engineering at the Gwangju Institute of Science and Technology (GIST). His research is dedicated to advancing circular economy technologies, with a focus on carbon, energy, and critical resources. Prior to GIST, he conducted postdoctoral research in the Department of Earth and Environmental Engineering at Columbia University in New York City, where he collaborated with Professor Ah-Hyung Alissa Park, exploring NOHMs and carbon mineralization for CO2 capture, utilization, and storage. Prof. Park obtained his Ph.D. in chemical and biomolecular engineering from KAIST in 2009 under the guidance of Prof. Huen Lee.
Journal
Accounts of Chemical Research
DOI
10.1021/acs.accounts.3c00406
Method of Research
Literature review
Subject of Research
Not applicable
Article Title
Advances in Nanomaterials for Sustainable Gas Separation and Storage: Focus on Clathrate Hydrates
Article Publication Date
21-Nov-2023
COI Statement
The authors declare no competing financial interest.




