This study is led by Xin-Wen Zhou (College of Materials and Chemical Engineering at China Three Gorges University).
Credit: Beijing Zhongke Journal Publising Co. Ltd.
This study is led by Xin-Wen Zhou (College of Materials and Chemical Engineering at China Three Gorges University).
The ethanol oxidation reaction (EOR) is an important anode reaction in direct ethanol fuel cells (DEFCs). It is well known that its ideal anode catalyst is noble metal platinum (Pt). However, its high cost, poor applicability, and poor anti-CO poisoning capacity make it difficult to promote scaled applications. Recent studies have shown that the preparation of new catalysts by combining precious metals with other non-noble metals and non-metals is an effective means to reduce costs and improve catalyst performance. Morphology control, surface engineering, improved carrier effect, and even a single atomic strategy can greatly improve the utilization of precious metals.
More and more studies have shown that the electrooxidation of ethanol in alkaline media is not only faster in kinetics but also has high activity and long-term stability. In the alkaline system, the lower-priced Pd (compared to the noble metal Pt) is used as the active material for catalytic oxidation of ethanol. Some studies have shown that the formation of noble metal-transition metal alloy catalysts, such as PdFe, PdCo, PdNi, PdCu, PdAu, PdAg, and AlNiCuPtPdCo, or noble metal/nonmetal composite material, not only reduces the amount of Pd in the catalyst but also plays an important role in improving the electrooxidation performance of ethanol. In recent years, breakthroughs have been made in anionic solid electrolyte membranes, greatly promoting the research on direct alkaline ethanol fuel cells (DAEFC), and its commercial application is expected to begin soon. The increase in the kinetics of the reaction of ethanol in an alkaline solution can be attributed to the fact that an increase in the pH of the electrolyte results in a negative shift of the working potential of 59 mV/pH, which changes the structure of the local electric double layer and the electric field distribution at the electrode/electrolyte interface, thus increasing the electrocatalytic activity. However, in an alkaline environment, many active transition metals (e.g., Fe, Co, Ni, Cu, etc.) can be stabilized, and their stability is enhanced by forming a multi-metal Pd-based catalyst. By adding a small amount of Cu2+ ions to the precursor, the spherical PtFe nanoparticles can be transformed into PtFeCu nanochains and exhibit enhanced oxidation properties of methanol.
Among them, PdFe-based catalysts have attracted the attention of researchers due to their special properties such as high electrical conductivity, excellent catalytic activity, and excellent durability and are often used as electrocatalysts for the oxygen reduction reaction (ORR) or anode oxidation reaction. The synergistic effect between the core and the shell will bring stress-strain effects to the surface and change the electronic structure of the surface metal, affecting the adsorption and reduction of oxygen. The phase transformation of the face-center cubic (fcc) to the face-center tetragonal (fct) of PdFe@Pd can indeed increase the active site of the catalyst, thus enhancing the catalytic performance of ORR. The assembled nano PdFe alloy film can be used as a highly efficient electrocatalyst for hydrogen evolution in acid and alkaline solutions due to the change in the valence electron structure of Pd and the increase in the electrochemical active area (ECSA).
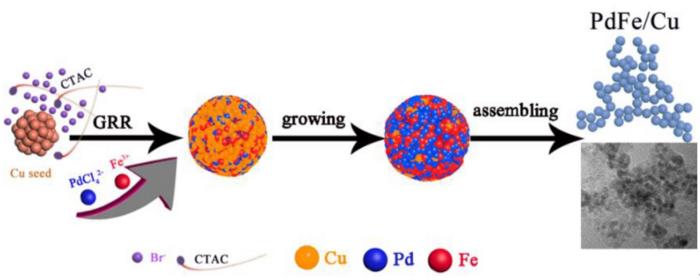
The galvanic replacement reaction (GRR) is a type of in-situ sacrificial template method used to prepare hollow nanocatalysts through a potential displacement method, widely employed in the field of material synthesis. Due to the difference in redox potential, the metal nanotemplate M (such as Co, Ni, Cu, etc.) with a low electrode potential reacts with precursors (such as Ptn+) with a high electrode potential, ultimately resulting in a hollow Pt or PtM catalyst. The GRR typically occurs in the stepwise reduction process, but a certain GRR process also takes place in the co-reduction method.
In this study, the stepwise co-reduction method was employed to design a PdFe/Cu nanocatalyst with a small particle size. Due to the strong reconstituted effect from the galvanic replacement reaction (GRR) process, the surface of the Cu seed crystal is oxidized, resulting in the formation of superfine PdFe/Cu nanoparticles. Additionally, the synergy and strain-stress effect between Pd, Fe, and Cu generated by this structural change alter the electronic structure of the nanoparticles. This alteration proves beneficial to the adsorption of ethanol molecules on the catalyst’s surface and the subsequent oxidation reaction, thereby improving the ethanol oxidation performance of the catalyst. At the same time, three other catalysts, namely PdFe, CuPdFe, and CuPdFe/Cu, were designed as controls. The conditions were optimized, and the electrooxidation performance of ethanol was tested. Finally, the catalyst with the optimal electrooxidation performance of ethanol under the alkaline system was determined.
See the article:
Highly active and durable PdFe/Cu nanocatalysts prepared by liquid phase synthesis for ethanol electrooxidation reaction
https://doi.org/10.1016/j.asems.2023.100075
Journal
Advanced Sensor and Energy Materials
DOI
10.1016/j.asems.2023.100075
Article Title
Highly active and durable PdFe/Cu nanocatalysts prepared by liquid phase synthesis for ethanol electrooxidation reaction
Article Publication Date
29-Aug-2023



