NATIONAL HEART CENTRE SINGAPORE PARTNERS MEDERA’S NOVOHEART TO DEVELOP ASIA’S FIRST BIOENGINEERED HUMAN HEART-IN-A-JAR WITH HEART FAILURE FOR PRECISION MEDICINE
Credit: National Heart Centre Singapore
NATIONAL HEART CENTRE SINGAPORE PARTNERS MEDERA’S NOVOHEART TO DEVELOP ASIA’S FIRST BIOENGINEERED HUMAN HEART-IN-A-JAR WITH HEART FAILURE FOR PRECISION MEDICINE
SINGAPORE/ BOSTON, MASSACHUSETTS, UNITED STATES, 21 November 2023 – National Heart Centre Singapore (NHCS) partners with Medera’s Novoheart to create the world’s first Asian patient-specific miniature human heart model with heart failure, known as “Human Heart-in-a-Jar”.
The novel creation will be made possible through Asian patients’ cell samples obtained from NHCS Biobank, a rich bio-repository of anonymised biospecimens and clinical data of patients with cardiovascular disease, and Novoheart’s proprietary technologies in the bioengineering of “Human Heart-in-a-Jar”. The collaboration will focus on creating the first Asian patient-specific mini-heart models capable of reproducing key features seen in patients that have heart failure with preserved ejection fraction (HFpEF).
Heart failure: A global pandemic
Heart failure (HF) is considered as a global pandemic, with higher prevalence in Southeast Asian countries where patients develop HF younger (by about 10 years) as compared in the Western regions, and associated with worse health outcomes1,2. In Singapore, HF is a leading cause of death, accounting for 17% of cardiac admissions locally3. HFpEF, a condition where the heart pumps normally but is too stiff to pump enough blood to the rest of the body, accounts for 50% of all heart failure cases4. With Singapore’s aging population and increasing prevalence of co-morbidities such as diabetes, obesity and hypertension, the number of patients developing HFpEF is set to rise.
Advancing in Heart Failure Care: Leveraging on World’s First mini-Heartᵀᴹ Technology
NHCS sees close to 3000 HF patients annually. As the leading national cardiac centre and the only heart and lung transplant centre in Singapore, NHCS provides comprehensive advanced HF management, from medical treatments to advanced therapies such as Extracorporeal Membrane Oxygenation (ECMO), as well as mechanical heart device implantations, and heart transplantations.
Beyond clinical care for HF, NHCS researchers have done substantial work in heart stem cell research in the last decade. More recently, the research team identified a new treatment for rhythm disorders5, and successfully created beating heart cells from patients with HF6. Indeed, while medical research in HFpEF has advanced tremendously, there have been a lack of accurate investigational models that mimic the clinical features of HFpEF, hampering preclinical testing of drug candidates for efficacy and development of treatment options for patients with HF.
Leveraging the centre’s experience and expertise in creating patient-specific beating heart cells, this partnership between NHCS and Medera’s Novoheart will accelerate the care for HFpEF patients. The bioengineering of the Asian patient-specific HFpEF mini-heart model, “Human Heart-in-a-Jar”, will be the first of its kind in Asia. Once successfully created, the “Human Heart-in-a-Jar” can reproduce key features seen in Asian HFpEF patients, including relaxation defects, fibrosis, and hypertrophy, and pave the way for precision medicine in HF.
With the world’s first mini-Heart technology platform, Novoheart has previously partnered with AstraZeneca to construct the first HFpEF mini-Heart by molecular induction. The results have led to an ongoing FDA-approved First-In-Human Gene Therapy clinical trial in the United States. Indeed, the US FDA Modernization Act 2.0 aims to accelerate innovation by ending the animal testing mandate and replacing it with scientifically superior, human-based technologies for improved accuracy and successes. The NHCS-Novoheart partnership, which focuses on the creation of Asian HFpEF mini-Heart by genetic means, aligns with this Act.
To accelerate progresses, Novoheart will also contribute proprietary commercial-grade hardware and software for automation to increase the throughput, accuracy and sensitivity of phenotypic and drug screening experiments using the engineered human heart-in-a-jar assays.
Next steps: Precision medicine for Heart Failure
The Asian patient-specific HFpEF mini-heart model will enable researchers to bridge the gap between animal models and clinical trials to accelerate the drug discovery process. With the increased accuracy and sensitivity of the human mini-heart model in responding to external factors, researchers will be able to conduct drug screening experiments to test patient-specific drugs before applying on actual patients.
“We are excited to partner with Novoheart on the HFpEF human mini-heart initiative which will provide a unique avenue for understanding the mechanisms of Asian patients to ultimately bring about new discoveries, potential therapeutics and better health outcomes for patients with heart failure,” said Professor Derek Hausenloy, Director, National Heart Research Institute Singapore (NHRIS), NHCS.
“This partnership with NHCS will cut across multiple biomedical and scientific disciplines as well as healthcare and academic sectors. We are laser-focused on the translation into tangible patient benefits in the new age of precision medicine for Asian heart patients,” said Dr. Ronald Li, Chief Executive Officer, Medera.
NHCS received a $5 million Industry Alignment Fund – Industry Collaboration Project (IAF-ICP) award from the Agency for Science, Technology and Research (A*STAR) to aid and accelerate drug discovery efforts for heart failure in Asian patients.
References:
- Heart failure in Southeast Asia:facts and numbers. ESC Heart Failure. 2015;2:46–9. doi: 10.1002/ehf2.12036
- Heart Failure and Multimorbidity in Asia, Curr Heart Fail Rep. 2023; 20(1): 24–32. doi: 10.1007/s11897-023-00585-2
- Chan WX, Lin W and Wong RCC. Transitional care to reduce heart failure readmission rates in South East Asia. Cardiac Failure Review 2016; 2: 85–9.
- Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017; 14:591–602. doi:10.1038/nrcardio.2017.65
- https://www.npm.sg/harnessing-genetics-to-understand-the-heart/
- https://www.astrazeneca.com/what-science-can-do/topics/disease-understanding/making-the-connection-targeting-multiple-mechanisms-in-heart-failure.html
Media Contact
|
Ms Belinda Lim Corporate Development National Heart Centre Singapore Tel: (65) 6704 2386 Email: [email protected] |
|
About the National Heart Centre Singapore
The National Heart Centre Singapore (NHCS) is a 185-bed national and regional referral centre for cardiovascular diseases, and the only heart and lung transplantation centre in Singapore. Providing a comprehensive range of cardiac care services from preventive to rehabilitative, NHCS’ clinical outcomes for heart attack treatment, balloon angioplasty with stenting, and coronary bypass surgery, are comparable to international benchmarks. An academic medical institution, NHCS actively trains and educates healthcare professionals to continuously raise the standards of cardiac care and conducts translational medical research with local and international collaborators to bring about better cardiac health for the community. For more information, please visit: www.nhcs.com.sg
Medera Biopharm, NovoHeart
Tel: 617-475-8541
Email: [email protected]
About Medera‘s Novoheart
Founded in 2014 and headquartered in Boston, Medera Biopharm (www.medera.bio) is a leading clinical-stage company dedicated to next-generation therapeutics for difficult-to-treat and incurable diseases. Medera has two subsidiaries, Novoheart (www.novoheart.com) and Sardocor (www.sardocor.com). Novoheart capitalizes on the world’s first and award-winning “mini-Heart” Technology for revolutionary disease modelling and drug discovery, enabling us to uniquely model human-specific diseases and discover therapeutic candidates free from species-specific differences. Before privatization by Medera, Novoheart was dually listed on the Toronto Stock Exchange and Frankfurt Stock Exchange. Sardocor (www.sardocor.com) aspires to create the shortest regulatory path to clinic for advancing next-generation cell and gene therapies. Building upon Novoheart’s bioengineered human tissue-based assays for disease modelling and drug discovery, Sardocor has developed one of the world’s largest gene- and cell-based therapeutic pipelines for a range of cardiac, vascular and muscular diseases including heart failure with preserved ejection fraction, Duchenne muscular dystrophy and pulmonary hypertension.
Annex
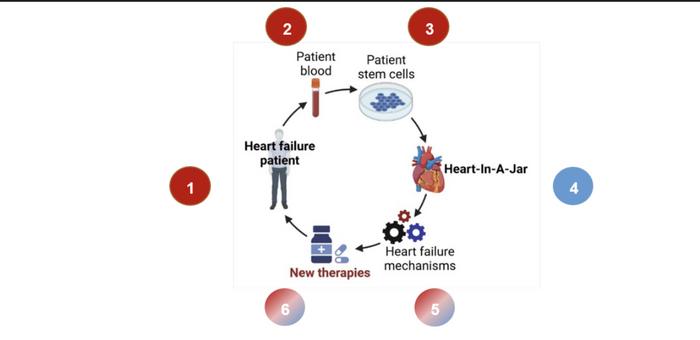
The illustration below summarises the collaborative efforts between NHCS and Novoheart to create Asia’s first bioengineered ‘Human Heart-in-a-Jar’:
- Stages 1 to 3: NHCS researchers retrieve a sample of the heart failure patient’s blood from NHCS Biobank to create patient-specific beating heart cells.
- Stages 4: With the patient-specific beating heart cells obtained in stage 3, Novoheart will create a miniature heart model, ‘Human Heart-In-A-Jar’, unique to this heart failure patient. The ‘Human Heart-In-A-Jar’ will reproduce key features seen in HFpEF patients, including relaxation defects, fibrosis, and hypertrophy.
- Stage 5 to 6: NHCS and Novoheart will discover new therapies for heart failure patients through testing patient-specific drugs on ‘Human Heart-in-a-Jar’ to ensure safety and efficacy before applying on actual patients.
Take a look at what beating heart cells look like here.
Find out more about Novoheart’s mini-heart platform here.
Glossary
|
Extracorporeal Membrane Oxygenation (ECMO) |
ECMO, a machine that takes over the functions of the heart and lungs, is a temporary life support technique to keep critically ill patients alive when conventional treatments have failed. |
|
Mechanical heart device |
A man-made artificial pump that takes over the pumping action of the heart to maintain blood circulation in the body. It may be a bridge-to-transplant, or destination therapy, for patients with advanced heart failure. |




