A research team, led by Tokyo Medical and Dental University(TMDU), identifies disease-associated variants in a rare case of RAD50 deficiency/Nijmegen breakage syndrome-like disorder
Credit: Department of Child Health and Development, TMDU
A research team, led by Tokyo Medical and Dental University(TMDU), identifies disease-associated variants in a rare case of RAD50 deficiency/Nijmegen breakage syndrome-like disorder
Tokyo, Japan – Many disorders are caused by genetic variants; to make matters worse, the genetic origin of most disorders remains unknown. Now, in a study recently published in the Journal of Clinical Immunology, researchers have shed light on the specific variants responsible for one rare and serious disorder: ‘RAD50 deficiency/Nijmegen breakage syndrome-like disorder’.
Together with MRE11 and NBN, RAD50 is one of three proteins that make up the ‘MRN complex’, which detects breaks in DNA and helps to initiate DNA repair. Because each of the three proteins is encoded by a separate gene, variants in any of the three genes can lead to altered functioning of the MRN complex. However, although MRE11 and NBN gene variants are known to cause other disorders, ataxia telangiectasia-like disorder and Nijmegen breakage syndrome, respectively, the pathological effects of RAD50 gene variants have remained somewhat unclear – until now.
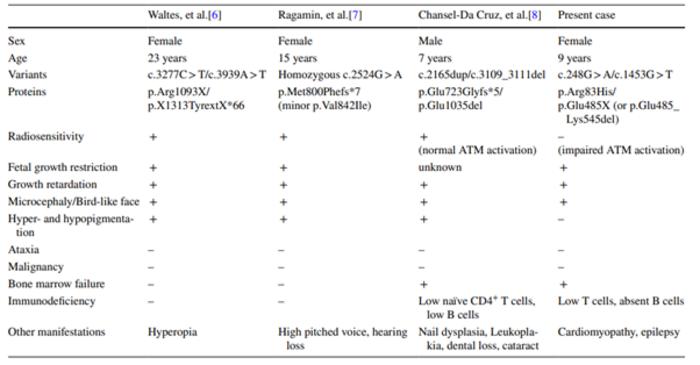
“When we looked at the literature, we realized that only three cases of RAD50 deficiency, which leads to symptoms similar to those of Nijmegen breakage syndrome, had been reported,” explains Masatoshi Takagi, lead author of the study. “Of these three, just one was reported to have RAD50 variants, with associated bone marrow failure and immunodeficiency.”
When the research team came across a patient with progressive bone marrow failure and immunodeficiency combined with Nijmegen breakage syndrome-like manifestations, they decided to perform whole-exome sequencing to see if they could identify any gene variants that might lead to the observed symptoms.
“We found two different RAD50 variants in our patient, each of which was inherited from one of her parents,” states Takagi. “We then tested the functional effects of these combined variants using fibroblast cells from the patient, which we grew in the lab.”
The functional experiments suggested that the patient’s RAD50 variants led to a loss of function of the RAD50 protein, and thus of the MRN complex. They also resulted in slower cell replication (i.e., mitosis), as expected. Interestingly, however, these variants did not cause hypersensitivity to radiation, unlike other known RAD50 variants.
“Together, the findings from our case and the three previously reported cases suggest that RAD50 deficiency/Nijmegen breakage syndrome-like disorder is characterized by growth retardation and microcephaly, which may coexist with bone marrow failure and immunodeficiency in some patients,” says senior author of the study Hirokazu Kanegane. “This disorder may therefore increase susceptibility to infectious diseases and immune-related conditions.”
Given the rarity of this disorder and our lack of knowledge about its genetic causes, the findings from this case are important. A better understanding of RAD50 and its effects on immunity can lead to improved diagnosis and treatment of patients with RAD50 deficiency.
###
The article, “Bone Marrow Failure and Immunodeficiency Associated with Human RAD50 Variants,” was published in the Journal of Clinical Immunology at DOI: 10.1007/s10875-023-01591-8
Journal
Journal of Clinical Immunology
DOI
10.1007/s10875-023-01591-8
Article Title
Bone Marrow Failure and Immunodeficiency Associated with Human RAD50 Variants




