Originally, the term “Sherpa” denoted a hill-tribe of Tibetan descent, but it has since become synonymous with guides on Mount Everest, the world’s highest and most rugged mountain. Much like these Sherpas, research into the demanding task of developing catalysts for hydrogen production is making substantial progress and has earned recognition as the featured cover article in a prominent international journal.
Credit: POSTECH
Originally, the term “Sherpa” denoted a hill-tribe of Tibetan descent, but it has since become synonymous with guides on Mount Everest, the world’s highest and most rugged mountain. Much like these Sherpas, research into the demanding task of developing catalysts for hydrogen production is making substantial progress and has earned recognition as the featured cover article in a prominent international journal.
Professor Yong-Tae Kim from the Department of Materials Science and Engineering and the Graduate Institute of Ferrous & Eco Materials Technology, and Kyu-Su Kim, a doctoral student from the Department of Materials Science and Engineering at Pohang University of Science and Technology (POSTECH), collaborated on a research project that offers a promising direction for the future development of catalysts for water electrolysis. Their study has garnered considerable academic attention and was showcased as the cover article in ACS Catalysis, an international journal in the field of chemistry.
Water electrolysis, a method for producing hydrogen from the abundant resource of water, emerges as an environmentally friendly technology that produces no carbon dioxide emissions. However, this process faces limitations due to its reliance on precious metal catalysts such as iridium (Ir), rendering it economically unfeasible. Researchers are actively exploring the development of catalysts in the form of metal alloys to address this challenge.
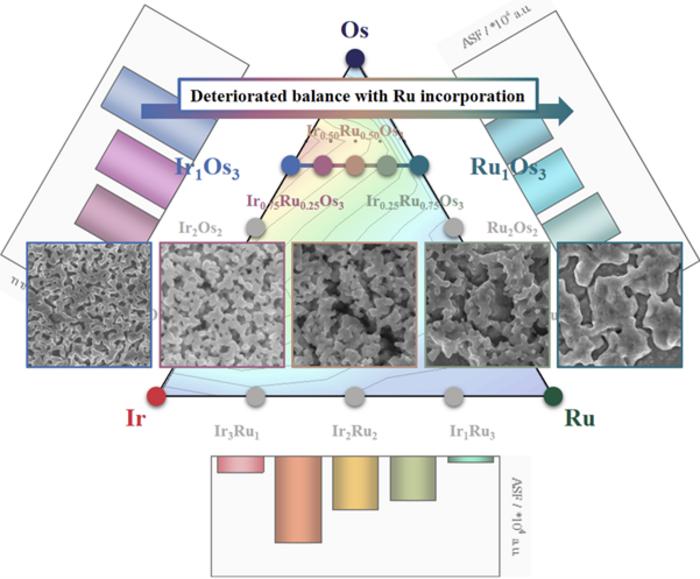
In the field of water electrolysis catalysis research, the primary catalysts under scrutiny are iridium, ruthenium (Ru), and osmium (Os). Iridium, despite its high stability, exhibits low activity and comes at a steep price. Conversely, ruthenium displays commendable activity and is a more cost-effective option compared to iridium, although it lacks the same level of stability. Osmium, on the other hand, readily dissolves under various electrochemical conditions, leading to the formation of nanostructures with an expanded electrochemical active surface area, thereby enhancing geometrical activity.
Initially, the research team developed catalysts using both iridium and ruthenium. By combining these metals, they successfully preserved the excellent attributes of each, resulting in catalysts that demonstrated improvements in both activity and stability. Catalysts incorporating osmium exhibited high activity due to the expanded electrochemical active surface area achieved through nanostructure formation. These catalysts retained the advantageous properties of iridium and ruthenium.
Subsequently, the team expanded their experimentation to include all three metals. The results showed a moderate increase in activity, but the dissolution of osmium had a detrimental effect, significantly compromising the structural integrity of iridium and ruthenium. In this series, the agglomeration and corrosion of nanostructures were accelerated, leading to a decline in the balance of catalytic performance.
Based on these findings, the research team has proposed several avenues for further catalyst research. First and foremost, they stress the need for a metric that can simultaneously evaluate both activity and stability. This metric, known as the activity-stability factor, was initially introduced by Kim’s research group in an international journal in 2017.
Additionally, the team advocates for the retention of superior catalyst properties even after the formation of nanostructures, in order to enhance the electrochemical active surface area of the electrocatalyst. They also highlight the importance of carefully selecting candidate materials that can effectively synergize when alloyed with other metals. The essence of this study lies not in presenting specific outcomes like the development of new catalysts, but rather in offering essential considerations for catalyst design.
Professor Yong-Tae Kim, who spearheaded the research, remarked, “This research marks the beginning of our journey, not the conclusion.” He shared his vision by stating, “We are dedicated to the continuous development of efficient water electrolysis catalysts based on the insights gained from this research.”
The study received support from the Future Materials Discovery Program of the National Research Foundation of Korea.
Journal
ACS Catalysis
DOI
10.1021/acscatal.3c01497
Article Title
Deteriorated Balance between Activity and Stability via Ru Incorporation into Ir-Based Oxygen Evolution Nanostructures
Article Publication Date
1-Sep-2023




