Philadelphia, November 2, 2023 – Major depressive disorder (MDD) is a widespread mental health condition that for many is disabling. It has long been appreciated that MDD has genetic as well as environmental influences. In a new study in Biological Psychiatry, published by Elsevier, researchers identify a gene that interacted with stress to mediate aspects of treatment-resistant MDD in an animal model.
Credit: Biological Psychiatry
Philadelphia, November 2, 2023 – Major depressive disorder (MDD) is a widespread mental health condition that for many is disabling. It has long been appreciated that MDD has genetic as well as environmental influences. In a new study in Biological Psychiatry, published by Elsevier, researchers identify a gene that interacted with stress to mediate aspects of treatment-resistant MDD in an animal model.
Jing Zhang, PhD, at Fujian Medical University and senior author of the study, said, “Emerging evidence suggests that MDD is a consequence of the co-work of genetic risks and environmental factors, so it is crucial to explore how stress exposure and risk genes co-contribute to the pathogenesis of MDD.”
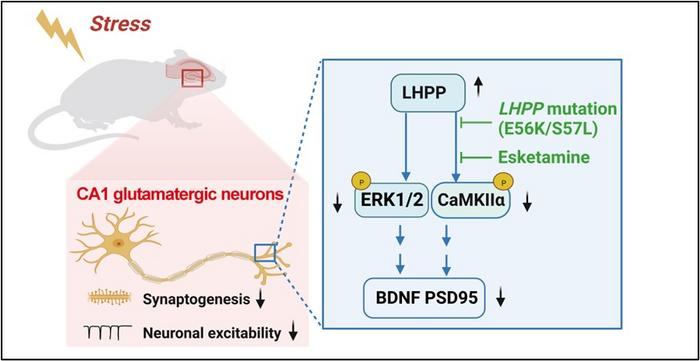
To do that, the authors used a mouse model of stress-induced depression called chronic social defeat stress (CSDS) in which mice are exposed to aggressor mice daily for two weeks. They focused on a gene called LHPP, which interacts with other signaling molecules at neuronal synapses. Increased expression of LHPP in the stressed mice aggravated the depression-like behaviors by decreasing expression of BDNF and PSD95 by dephosphorylating two protein kinases, CaMKIIα and ERK, under stress exposure.
Dr. Zhang noted, “Interestingly, LHPP mutations (E56K, S57L) in humans can enhance CaMKIIα/ERK-BDNF/PSD95 signaling, which suggests that carrying LHPP mutations may have an antidepressant effect in the population.”
MDD is an extremely heterogeneous condition. Differences in the types of depression experienced by people influence the way they respond to treatment. A large subgroup of people with depression fail to respond to standard antidepressant medications and have “treatment-resistant” symptoms of depression. These patients often respond to different medications, such as ketamine or esketamine, or to electroconvulsive therapy. Notably, esketamine markedly alleviated LHPP-induced depression-like behaviors, whereas the traditional drug fluoxetine did not, suggesting that the mechanism might underlie some types of treatment-resistant depression.
John Krystal, MD, Editor of Biological Psychiatry, said of the work, “We have limited understanding of the neurobiology of treatment-resistant forms of depression. This study identifies a depression risk mechanism for stress-related behaviors that fail to respond to a standard antidepressant but respond well to ketamine. This may suggest that the risk mechanisms associated with the LHPP gene shed light on the poorly understood biology of treatment-resistant forms of depression.”
Dr. Zhang added, “Together, our findings identify LHPP as an essential player driving stress-induced depression, implying targeting LHPP as an effective strategy in MDD therapeutics in the future.”
Journal
Biological Psychiatry
DOI
10.1016/j.biopsych.2023.08.026
Method of Research
Experimental study
Subject of Research
Animals
Article Title
LHPP in glutamatergic neurons of the ventral hippocampus mediates depression-like behavior by dephosphorylating CaMKIIα and ERK
Article Publication Date
9-Sep-2023
COI Statement
The authors’ affiliations and disclosures of financial and conflicts of interests are available in the article.
John H. Krystal, MD, is Chairman of the Department of Psychiatry at the Yale University School of Medicine, Chief of Psychiatry at Yale-New Haven Hospital, and a research psychiatrist at the VA Connecticut Healthcare System. His disclosures of financial and conflicts of interests are available at http://www.biologicalpsychiatryjournal.com/content/bps-editorial-disclosures.




