A finer glass tube and a more exacting technique with a refined procedure can reduce the likelihood of transmitting mitochondrial disease during assisted reproduction, according to a new study publishing October 5th in the open access journal PLOS Biology by Qifeng Lyu and colleagues of Shanghai Jiao Tong University School of Medicine, China. The study may improve the odds of having a healthy baby for women who carry mutations in their mitochondrial DNA.
Credit: Xiaoyu Liao, Liao X et al., 2023, PLOS Biology, CC-BY 4.0 (https://creativecommons.org/licenses/by/4.0/)
A finer glass tube and a more exacting technique with a refined procedure can reduce the likelihood of transmitting mitochondrial disease during assisted reproduction, according to a new study publishing October 5th in the open access journal PLOS Biology by Qifeng Lyu and colleagues of Shanghai Jiao Tong University School of Medicine, China. The study may improve the odds of having a healthy baby for women who carry mutations in their mitochondrial DNA.
Mitochondria, the cell’s major center for energy production, have their own DNA that encodes several dozen essential genes. Mutations in these genes can cause disease, including a type of optic neuropathy, metabolic disorders, and muscle disease. These diseases are transmitted only via the mother, because all of an embryo’s mitochondria come from the egg, not the sperm.
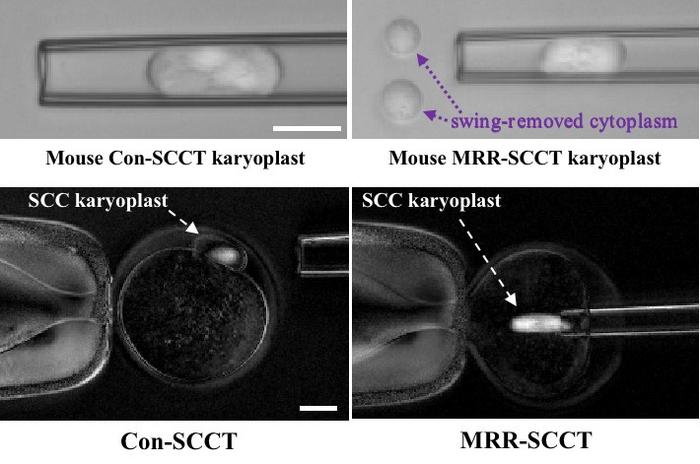
In a mother carrying such mutations, one technique that is used to reduce the chances of disease transmission is spindle-chromosomal complex transfer (SCCT), in which the nuclear chromosomes are removed from an unfertilized egg while they are arranged along a structure called the spindle complex, and transferred to a donor egg cell that has healthy mitochondria. Unfortunately, some disease-carrying maternal mitochondria often get swept up along with the spindle complex. After egg fertilization, during embryo formation, some of them may multiply to form a significant fraction of the mitochondria in some tissues, and so cause disease.
To reduce the likelihood of mitochondrial transfer during SCCT, the authors made three key changes to the standard SCCT protocol. First, they transferred sperm into the egg cell before removing the spindle-chromosomal complex, rather than waiting until after removal, since previous studies have shown that manipulation itself can prematurely activate the meiotic process, which is normally arrested until fertilization.
Second, after extracting the spindle-chromosomal complex from the egg, they transferred it into an even narrower-diameter tube (12 micrometers for mice, 10 micrometers for humans), in order to begin to squeeze cytoplasm away from the complex. Finally, they aspirated the cytoplasm/complex into a viscous chemical medium, and manipulated the pipette to further separate the complex from the cytoplasm, a process the authors called “swinging away” the carried cytoplasm. With the complex now largely devoid of cytoplasm, they transferred it into the waiting enucleated egg cell.
In mice, the procedure resulted in normal development of embryos and healthy offspring, with no evidence of expansion of donor-egg mitochondrial population in offspring tissues. In human oocytes, the level of mitochondria carried over from the donor egg was about 4% of that from the standard protocol, with no evidence of damaged chromosomes, and high rates of normal early embryonic development.
“This novel protocol should reduce mitochondrial DNA carryover to the lowest level currently possible,” Lyu said, reducing the risk of development of genetic disease in the offspring of maternal carriers. “However, we have not been able to conclude yet that this procedure is adequately safe in the clinic for preventing the transmission of inherited mitochondrial DNA diseases,” and further research will be needed before clinical translation of this procedure.
Lyu adds, “We highlight that the MRR-SCCT strategy demonstrated not only efficient residue removal of mtDNA but also fantastic embryo development in mouse and human oocytes.”
#####
In your coverage, please use this URL to provide access to the freely available paper in PLOS Biology: http://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3002313
Citation: Liao X, Li W, Lin K, Jin W, Zhang S, Wang Y, et al. (2023) Significant decrease of maternal mitochondria carryover using optimized spindle-chromosomal complex transfer. PLoS Biol 21(10): e3002313. https://doi.org/10.1371/journal.pbio.3002313
Author Countries: China
Funding: see manuscript
Journal
PLoS Biology
DOI
10.1371/journal.pbio.3002313
Method of Research
Experimental study
Subject of Research
Cells
COI Statement
Competing interests: The authors have declared that no competing interests exist.




