In the relentless battle against antibiotic-resistant superbugs, science continues to unveil ingenious strategies to address their vulnerability. Like other bacteria, superbugs have a unique weakness – their dependence on iron for growth and survival. Iron serves as an essential nutrient that bacteria utilise for various cellular processes, including DNA replication, energy production, and other vital functions. In essence, iron is like a ‘food’ for bacteria.
Credit: Nature Communications
In the relentless battle against antibiotic-resistant superbugs, science continues to unveil ingenious strategies to address their vulnerability. Like other bacteria, superbugs have a unique weakness – their dependence on iron for growth and survival. Iron serves as an essential nutrient that bacteria utilise for various cellular processes, including DNA replication, energy production, and other vital functions. In essence, iron is like a ‘food’ for bacteria.
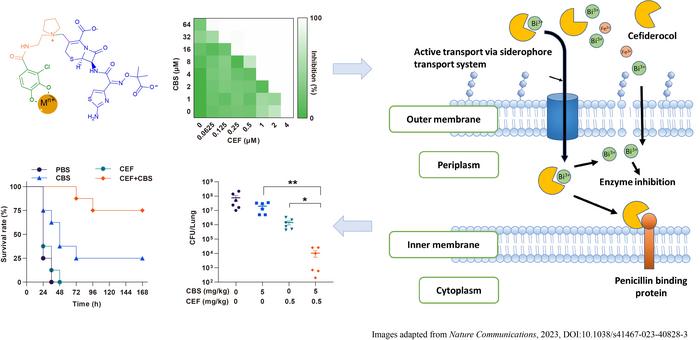
Building upon this understanding, a research team led by Professor Hongzhe SUN from the Department of Chemistry, The University of Hong Kong (HKU), introduced a ‘Dual Trojan Horse’ strategy, where a metal-based-drug and sideromycins, a class of antibiotic structurally resembling iron, work together in combating antibiotic-resistant bacteria. This approach allows these antibiotics to be delivered into bacterial cells through a pathway that mimics iron uptake. When bacteria encounter sideromycins, they are deceived into believing they are acquiring iron, prompting them to usher these compounds into their cells. This strategy not only enhances the effectiveness of sideromycins but also prolongs their lifespan, marking a significant advancement in our battle against antibiotic resistance. These promising results were successfully replicated in a live mice model, introducing an innovative strategy to combat antimicrobial resistance, offering hope in the fight against superbugs in clinic. These findings have recently been published a in Nature Communications entitled ‘Metallo-sideromycin as a dual functional complex for combating antimicrobial resistance (AMR)’.
‘We are short of new antibiotics, and infection caused by resistant bacteria (i.e. superbugs) may lead to another pandemic. We have uncovered a dual Trojan Horse strategy to restore antibiotics activity, such as cefiderocol, and hope to provide a novel arsenal for combating antimicrobial resistance,’ commented Professor Sun.
Research Background
Antimicrobial resistance (AMR) in bacterial infections has emerged as a significant global health concern. The overuse and misuse of existing antibiotics have accelerated the acquired drug resistance in bacteria, resulting in resistance to almost all antibiotics used in clinical settings across various bacteria strains.
Gram-negative bacterial infections, such as those caused by Pseudomonas aeruginosa, pose significant challenges in treatment due to their complicated structure. For example, the high resistance of P. aeruginosa against conventional antibiotics can be attributed in part to the limited permeability of the outer membrane (OM) and the expression of ‘efflux pump’, specialised proteins within bacteria that actively remove antibiotics, thus reducing their effectiveness. These factors collectively impede the accumulation of antibiotics at the bacterial target site.
Gram-negative bacteria, including Pseudomonas aeruginosa, can cause a range of infections in humans. These infections often affect the respiratory system, leading to pneumonia or lung infections, as well as urinary tract infections. They can also lead to skin and soft tissue infections, bloodstream infections (sepsis), and infections in wounds or surgical sites. In severe cases, these infections can be particularly challenging to treat due to the bacteria’s resistance to antibiotics, making them a significant health concern. For these reasons, there is now an urgent need for both new antibiotic discovery and other modifications or strategies to enhance or prolong the antibacterial activity of existing clinical antibiotics.
Sideromycin is a novel type of antibiotic where the parent antibiotics or prodrug incorporates a siderophore molecule that utlises iron transport system for delivery. This incorporation enables the active transport of the antibiotic into bacterial cell through nutrient pathways. Cefiderocol (FetrojaÒ) is a recently FDA-approved sideromycin antibiotic in 2019. The antibacterial activity of cefiderocol is improved under the iron-deficient condition because of the enhanced uptake of cefiderocol, with a component of catechol, which coordinate with iron and facilitate the transportation of cefiderocol-iron complex in P. aeruginosa.
Although the frequency of resistance of P. aeruginosa to cefiderocol is much lower than its parent antibiotic ceftazidime, the resistance to cefiderocol was developed inevitably in several Gram-negative bacteria strains recently, for example, in Carbapenem-Resistant Escherichia coli strains and Acinetobacter baumannii in the burned infections. Resistance to cefiderocol was related to the production of β-lactamases, siderophore receptor mutations, expression of efflux pump and the combination of these mechanisms.
Metal compounds have been used as promising antimicrobial agents for years and show low resistance frequency since they are multi-targeted modes of action. Bismuth (Bi3+) compounds have exhibited potent antibacterial properties against bacterial that have become resistant to a variety of antibiotics. These bismuth compounds act as versatile inhibitors of a group of enzymes called metallo-β-lactamase inhibitors, which are involved in antibiotic resistance. Gallium(Ga3+) also offers antibacterial activities by disrupting Iron (Fe3+)uptake system and iron homeostasis.
Interestingly, catecholate siderophores exhibit exceptionally high affinity not only to iron (Fe3+), but also to metals like bismuth (Bi3+) and gallium (Ga3+). These metals behave similarly to iron when they link up with catecholate molecules. These special catechol-metal combinations have been observed to do two things: they can compete with iron to get inside bacterial cells, and they can imitate iron in biological systems, disrupting important iron functions. Thus, the team propose a dual ‘Trojan Horse’ strategy to ‘sneak in’ the antibiotic sideromycins and metal ions simultaneously through siderophore receptor, the same pathways that bacteria use to grab nutrients, leading to synergistic effect against bacterial infections.
Key findings
In this study, the team demonstrated a bismuth drug (CBS) could enhance the potency of cefiderocol against P. aeruginosa in both laboratory experiment (in vitro) and live animal test (in vivo). This enhancement included improved efficacy against biofilm formation by cefiderocol, suppression of the development of high-level bacterial resistance to cefiderocol, and restoration of the efficacy of cefiderocol against resistant P. aeruginosa strains, including those isolated from clinical cases involving real patients.
Such phenomena are likely due to the competition of Bi3+ with Fe3+ to cefiderocol, which leads to decreased uptake of Fe3+ and increased uptake of antimicrobial Bi3+/Ga3+. This competition disrupts the integrity of bacterial membrane, making antibiotic more permeable.
The in vitro interaction of Bi3+ with cefiderocol was confirmed by both UV-vis spectroscopy and MS spectrometry, analytical techniques which confirmed the interaction between Bi3+ and cefiderocol, resulting in the formation of a 1:1 complex of Bi3+-cefiderocol. The metallo-sideromycin might not only improve the efficiency of sideromycin, but also prolong the effective life span of this type of antibiotics. Their animal studies have further validated the efficacy of the approach. It is worth of further investigation of other sideromycins and metals, to thoroughly explore the potentials of metallo-sideromycins in treating infections caused by drug-resistant bacterial pathogens. The research team has filed a patient for the discovery.
About the research team
This study was done jointly by the Department of Chemistry, Department of Microbiology and Carol Yu Centre for Infection, The University of Hong Kong. Ms Chenyuan WANG and Dr Yushan XIA are the co-first authors of this paper. Other members of participating in the research include Dr Hongyan LI, Dr Patrick H TOY, Dr Runming WANG, postgraduate student Ms Jingru LI, and Mr Chun-Lung CHAN of Department of Chemistry, Professor Richard Yi-Tsun KAO of Department of Microbiology, Professor Pak-Leung HO of Carol Yu Centre for Infection. This research was supported by the Research Grants Council of Hong Kong SAR (R7070-18, 17308921, 2122-7S04), the Health and Medical Research Fund of the Health Bureau of Hong Kong SAR (CID HKU1-13) and The University of Hong Kong (URC (202107185074) and Norman & Cecilia Yip Foundation).
About Professor Hongzhe Sun
Professor Hongzhe Sun is the Norman & Cecilia Yip Professor in Bioinorganic Chemistry and Chair Professor of Chemistry at The University of Hong Kong. His research focuses on metalloproteomics and metallomics, the discovery of antimicrobial and antiviral agents, and inorganic chemical biology. Dr Hongyan Li is a Research Assistant Professor in the Department of Chemistry at The University of Hong Kong.
To view the research paper ‘Metallo-sideromycin as a dual functional complex for combating antimicrobial resistance’, please visit: https://www.nature.com/articles/s41467-023-40828-3
Image download and caption: https://www.scifac.hku.hk/press
For media enquiries, please contact Ms Casey To, External Relations Officer (tel: 39174948; email: [email protected] / Ms Cindy Chan, Assistant Director of Communications of HKU Faculty of Science (tel: 3917 5286; email: [email protected]).
Journal
Nature Communications
DOI
10.1038/s41467-023-40828-3
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Metallo-sideromycin as a dual functional complex for combating antimicrobial resistance
Article Publication Date
1-Sep-2023




