By Benjamin Boettner
Credit: Wyss Institute at Harvard University
By Benjamin Boettner
(BOSTON) — Cancer immunotherapy has brought major improvement in patient survival and quality of life, especially with the success of adoptive T cell and immune checkpoint inhibitor therapies. Unfortunately, in contrast to different blood cancers, the effectiveness of adoptive T cell therapies in the treatment of solid tumors, which comprise about 90% of all tumors, has been very limited because of several formidable barriers.
In adoptive T cell therapies, a patient’s T cells with cytotoxic potential are engineered outside the body so that they can bind specific features (antigens) on the surface of tumor cells, which converts them into tumor-killing cells. However, after being reinfused into the donor patient’s blood circulation, they have to travel long distances to reach a solid tumor with only a fraction of them ever arriving there. On-site, they need to infiltrate the often difficult-to-penetrate tumor mass, while their cytotoxic activity is suppressed by tumor cells and their surrounding tissue microenvironment. In addition, the further solid tumors grow, the more heterogenous their cell composition becomes, which also includes tumor cells’ repertoire of surface antigens, and thus allows them to “escape” the attack of adoptively transferred T cells.
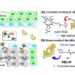
Now, a team of immune-engineers at the Wyss Institute for Biologically Inspired Engineering at Harvard University and Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) have developed a novel biomaterials-based immunotherapy approach named SIVET (short for “synergistic in situ vaccination enhanced T cell”) that has the potential to break down these barriers. The injectable biomaterial enables both: the local delivery of antigen-specific adoptively transferred T cells directly to tumor sites and their prolonged activation, as well as a broader engagement of the host immune system to provide much longer-lasting anti-tumor effects against tumor cells carrying new antigens. Validated in mice carrying melanomas, a particularly aggressive type of solid tumor, SIVET enabled the fast shrinking of tumors and long-term protection against them. The findings are published in Nature Communications.
“In the SIVET approach, we essentially combined fast-acting adoptive T cell therapy with long-term protective cancer vaccine technology in a locally delivered integrated biomaterial. Advancing this approach towards patient settings could help addresses several limitations of current immunotherapies and offers new inroads into the treatment of solid tumors,” said senior author David Mooney, Ph.D., who is a Founding Core Faculty member at the Wyss Institute and the Robert P. Pinkas Family Professor of Bioengineering at SEAS. Mooney leads the Wyss Institute’s Immunomaterials Platform and co-leads the NIH-funded Immuno-Engineering to Improve Immunotherapy (i3) Centercoordinated at the Wyss Institute and focused on creating biomaterials-driven approaches to enable anti-cancer immunotherapy in solid tumor settings.
Biomaterial convergences
In extensive previous work, Mooney’s team had pioneered biomaterial-based cancer vaccines that are able to program key immune-orchestrating dendritic cells, known as antigen-presenting cells (APCs), into tumor-fighting cells in vivo. Despite the cancer vaccines being able to provide broad therapeutic and prophylactic benefits, their tumor-directed effects take time to manifest in the body. On the other hand, patient-specific adoptively transferred T cells are ready-made to attack tumor cells upon first contact but produce rather short-lived responses.
“Our new platform fully leverages our expertise with adoptive T cell and cancer vaccine technologies. Combining the best of these two worlds in a multi-pronged biomaterial-based approach allows the fast debulking of existing tumor masses while engaging the immune system on a much deeper level through the localized delivery, concentration, and activation of diverse tumor-fighting immune cells,” said co-first author Kwasi Adu-Berchie, Ph.D., who completed his Ph.D. in Mooney’s lab and is currently a Translational Immunotherapy Scientist at the Wyss Institute.
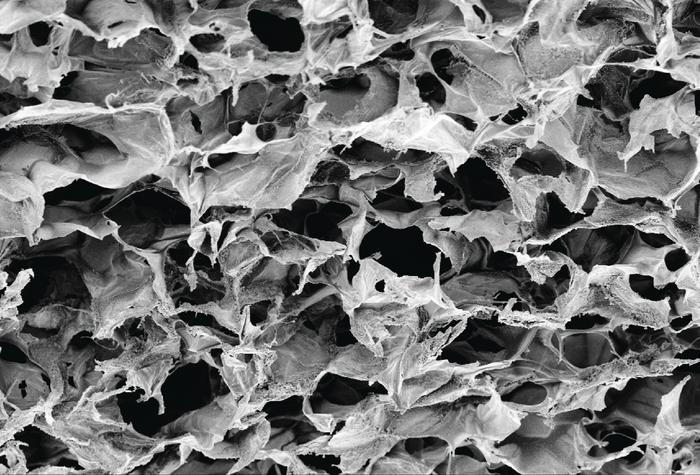
Adu-Berchie, Mooney, and the team developed a cryogel biomaterial that contains collagen and alginate polymers cross-linked into a 3-dimensional porous scaffold. While the alginate provides the biomaterial with structural support, collagen serves to provide ligands needed for T cell trafficking. Following injection of the engineered T cell depot close to a tumor site, the compressed biomaterial recovers its original shape and starts releasing the cytokine interleukin 2 (IL2) to facilitate the expansion of the delivered T cells, which move out of the biomaterial and onto the tumor to carry out an attack.
In addition, the biomaterial releases a second cytokine, abbreviated as GMCSF, which attracts host APCs into the porous scaffold that then also become concentrated and activated with the help of an adjuvant molecule known as CpG close to the tumor. The activated APCs also infiltrate the tumor mass where they take up new antigens created by dying tumor cells that disintegrate as a result of the T cell attack. The APCs then migrate to nearby lymph nodes where they orchestrate a broader vaccine response by presenting processed antigens to other immune cell types, including other cytotoxic T cells that attack the tumor in consecutive waves, as well as memory T cells that stand by for future tumor recurrences.
The researchers investigated SIVET in a mouse model carrying melanoma tumors and found that the multi-functional biomaterial enabled better control over the tumors than the same adoptively transferred T cells injected directly into the tumor site or infused into the blood stream of the animals. SIVETs enabled the delivered T cells to remain active longer and minimized the exhaustion of all T cells in the tumor microenvironment when compared to control conditions.
“Through their vaccine component, SIVETs trained the immune system to reject melanoma tumors for significantly prolonged periods of time, and thus allowed the animals to survive for significantly longer than animals that received any of our control treatments. This likely was facilitated by the biomaterial’s ability to prevent the growth of tumor cells that escape the attack of adoptively transferred T cells due to their loss of the initially targeted antigen,” said Adu-Berchie. “Identifying a tumor-specific antigen against which potent donor-specific T cells can be generated for adoptive transfer could provide SIVETs with enough to go on to initiate a tumor attack on a much broader front and scale.
“This study is a beautiful convergence of two powerful immunotherapy approaches that are programmed in the body to synergize with each other. This work once again demonstrates the power of taking an unconventional trans-disciplinary approach – in this case, combining strategies from materials science and tissue engineering with immunology – to create novel and more powerful therapeutics for the eradication of solid cancers,” said Wyss Founding Director Donald Ingber, M.D., Ph.D., who is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children’s Hospital, and the Hansjörg Wyss Professor of Bioinspired Engineering at SEAS.
The study is also authored by other past and present members of Mooney’s group, including Joshua Brockman, Yutong Liu, Tania To, David Zhang, Alexander Najibi, Yoav Binenbaum, Alexander Stafford, Nikolaus Dimitrakakis, Miguel Sobral, and Maxence Dellacherie. It was supported by grants from the National Institutes of Health (award #U54 CA244726 and #U01 CA214369), National Science Foundation (award #MRSEC DMR-1420570), and Food and Drug Administration (award #R01 FD006589), as well as the National Cancer Institute (award #5K00CA234959).
PRESS CONTACTS
Wyss Institute for Biologically Inspired Engineering at Harvard University
Benjamin Boettner, [email protected], +1 617-432-8232
###
The Wyss Institute for Biologically Inspired Engineering at Harvard University (www.wyss.harvard.edu) is a research and development engine for disruptive innovation powered by biologically-inspired engineering with visionary people at its heart. Our mission is to transform healthcare and the environment by developing ground-breaking technologies that emulate the way Nature builds and accelerate their translation into commercial products through formation of startups and corporate partnerships to bring about positive near-term impact in the world. We accomplish this by breaking down the traditional silos of academia and barriers with industry, enabling our world-leading faculty to collaborate creatively across our focus areas of diagnostics, therapeutics, MedTech, and sustainability. Our consortium partners encompass the leading academic institutions and hospitals in the Boston area and throughout the world, including Harvard’s Schools of Medicine, Engineering, Arts & Sciences and Design, Beth Israel Deaconess Medical Center, Brigham and Women’s Hospital, Boston Children’s Hospital, Dana–Farber Cancer Institute, Massachusetts General Hospital, the University of Massachusetts Medical School, Spaulding Rehabilitation Hospital, Boston University, Tufts University, Charité – Universitätsmedizin Berlin, University of Zürich, and Massachusetts Institute of Technology.
The Harvard John A. Paulson School of Engineering and Applied Sciences (http://seas.harvard.edu) serves as the connector and integrator of Harvard’s teaching and research efforts in engineering, applied sciences, and technology. Through collaboration with researchers from all parts of Harvard, other universities, and corporate and foundational partners, we bring discovery and innovation directly to bear on improving human life and society.
Journal
Nature Communications
DOI
10.1038/s41467-023-39330-7
Method of Research
Experimental study
Subject of Research
Animals
Article Title
Adoptive T cell transfer and host antigen-presenting cell recruitment with cryogel scaffolds promotes long-term protection against solid tumors
Article Publication Date
15-Jun-2023