EVANSTON, Ill. — Nearly 700,000 people in the United States die from heart disease every year, and one-third of those deaths result from complications in the first weeks or months following a traumatic heart-related event.
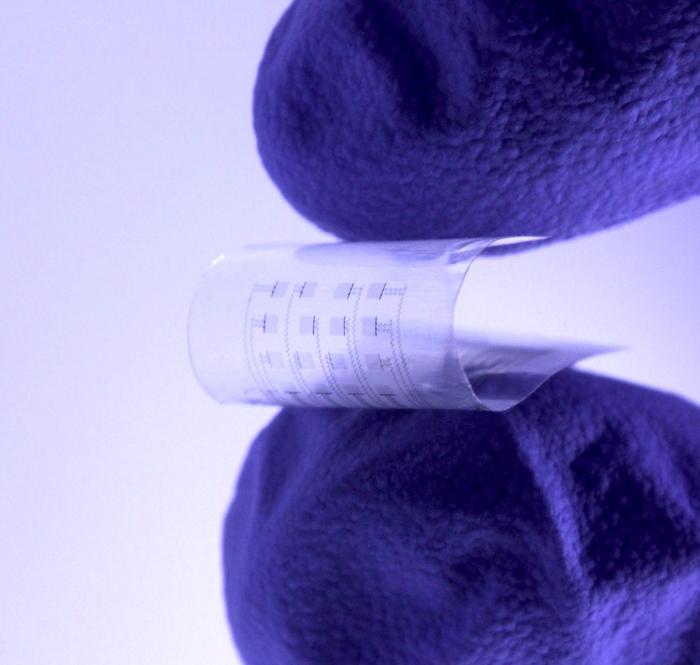
Credit: Northwestern University/George Washington University
- New device shows promise in small animal studies
- Not only can it restore normal heart rhythms, it also can show which areas of the heart are functioning well and which areas are not
- After the device is no longer needed, it harmlessly dissolves inside the body, bypassing the need for extraction
EVANSTON, Ill. — Nearly 700,000 people in the United States die from heart disease every year, and one-third of those deaths result from complications in the first weeks or months following a traumatic heart-related event.
To help prevent those deaths, researchers at Northwestern and George Washington (GW) universities have developed a new device to monitor and treat heart disease and dysfunction in the days, weeks or months following such events. And, after the device is no longer needed, it harmlessly dissolves inside the body, bypassing the need for extraction.
About the size of a postage stamp, the soft, flexible device uses an array of sensors and actuators to perform more complicated investigations than traditional devices, such as pacemakers, can accomplish. Not only can it be placed on various sections of the heart, the device also continuously streams information to physicians, so they can remotely monitor a patient’s heart in real time. The device also is highly transparent, allowing physicians to observe specific heart regions to make a diagnosis or provide a treatment.
The research will be published on Wednesday (July 5) in the journal Science Advances.
“Several serious complications, including atrial fibrillation and heart block, can follow cardiac surgeries or catheter-based therapies,” said Northwestern’s Igor Efimov, an experimental cardiologist who co-led the study. “Current post-surgical monitoring and treatment of these complications require more sophisticated technology than currently available. We hope our new device can close this gap in technology. Our transient electronic device can map electrical activity from numerous locations on the atria and then deliver electrical stimuli from many locations to stop atrial fibrillation as soon as it starts.”
“Many deaths that occur following heart surgery or a heart attack could be prevented if doctors had better tools to monitor and treat patients in the delicate weeks and months after these events take place,” added GW’s Luyao Lu, who co-led the work with Efimov. “The tool developed in our work has great potential to address unmet needs in many programs of fundamental and translational cardiac research.”
Efimov is a professor of biomedical engineering at Northwestern’s McCormick School of Engineering and professor of medicine at Northwestern University Feinberg School of Medicine. Lu is an assistant professor of biomedical engineering at GW.
This work builds on Efimov’s previous work to develop cardiac implants to monitor and temporarily pace the heart. In 2021, Efimov and Northwestern professor John A. Rogers introduced the first-ever transient pacemaker, published in Nature Biomedical Engineering. Then, earlier this year, Efimov’s team unveiled a graphene “tattoo” for treating cardiac arrhythmia, published in Advanced Materials.
“After heart surgeries, surgeons sometimes insert temporary wires, which are connected to external current generators, to provide electrical stimulation during temporary heart block caused by the surgery,” Efimov said. “Recently, we developed a bioresorbable pacemaker to replace such a wire. Post-operative atrial fibrillation requires a more complicated approach based on a multi-electrode array for sensing and stopping atrial fibrillation. Now, we present a novel technology to achieve this goal.”
Tested in small animal models, the new device provides functions beyond those of a traditional pacemaker. While a pacemaker only can provide one overall picture of the heart (whether or not the heart is beating), the transient device provides a more nuanced picture. Not only can it restore normal heart rhythms, it also can show which areas of the heart are functioning well and which areas are not. The device’s transparent nature also allows researchers to optically map many important cardiac physical parameters through the device to better study heart function and heart disease mechanisms.
After a clinically relevant period, the device — which is made of biocompatible materials approved by the U.S. Food and Drug Administration — simply dissolves into benign products. Similar to absorbable stitches, the device degrades and then completely disappears through the body’s natural biological processes. The device’s bioresorbable nature could reduce healthcare costs and improve patient outcomes by avoiding complications from surgical extraction and lowering infection risks.
The study, “Soft, bioresorbable, transparent microelectrode arrays for multimodal spatiotemporal mapping and modulation of cardiac physiology,” was supported by the National Science Foundation and the National Institutes of Health.
Journal
Science Advances
DOI
10.1126/sciadv.adi0757
Method of Research
Experimental study
Subject of Research
Animals
Article Title
Soft, bioresorbable, transparent microelectrode arrays for multimodal spatiotemporal mapping and modulation of cardiac physiology
Article Publication Date
5-Jul-2023



