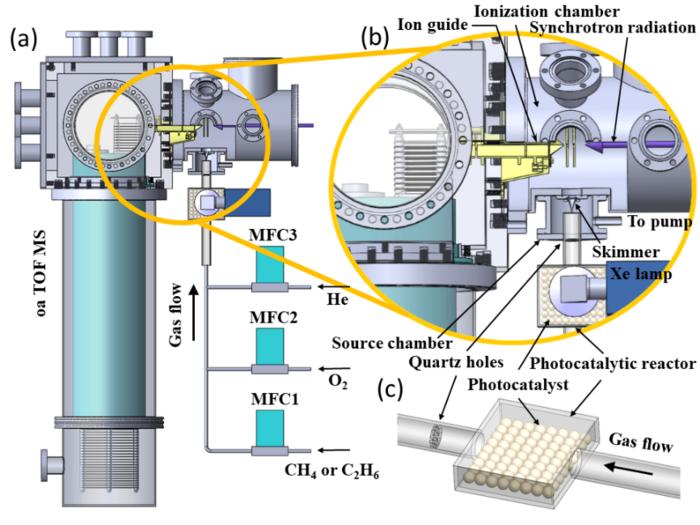
Prof. PAN Yang and Associate Researcher LIU Chengyuan, researchers from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS), have achieved significant progress in detecting intermediates in methane photocatalytic reactions. Their technique, Synchronous Radiation Photoionization Mass Spectrometry (SR-PIMS), allows for in-situ detection of active intermediates. The findings were published in the prestigious chemistry journal Angewandte Chemie International Edition on May 23rd.
Credit: Schematic diagram of the in situ photocatalytic mass spectrometry unit
Prof. PAN Yang and Associate Researcher LIU Chengyuan, researchers from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS), have achieved significant progress in detecting intermediates in methane photocatalytic reactions. Their technique, Synchronous Radiation Photoionization Mass Spectrometry (SR-PIMS), allows for in-situ detection of active intermediates. The findings were published in the prestigious chemistry journal Angewandte Chemie International Edition on May 23rd.
Methane, a clean and abundant carbon resource derived from natural gas, shale gas, and methane hydrates, holds great potential for energy, chemical, and environmental sciences. Its direct conversion into valuable chemicals through photocatalysis has gained attention. However, the lack of in-situ detection methods, especially for active intermediates like free radicals, has been a challenge.
Prof. Pan Yang and Assoc. Researcher Liu Chengyuan, along with their team, utilized synchrotron radiation photoionization technology to develop a mass spectrometry characterization technique for capturing gas-phase active intermediates in photocatalytic reactions. By combining a rectangular photocatalytic reactor with synchrotron radiation as the light source, this technique can detect intermediates within a few hundred microseconds during the reaction process.
In the experiments, Ag-ZnO was used as the catalyst, and the photocatalytic reaction was initiated by controlling the flow of methane, oxygen, and helium. Gas-phase intermediates and stable products were detected through mass spectrometry analysis after detachment from the catalyst surface. The experimental results directly observed the gas-phase methyl radicals (•CH3) generated by photo-generated holes (O-) and found a significant enhancement in their production due to co-adsorbed oxygen molecules. Additionally, methanol (CH3OH), formaldehyde (HCHO), and methoxy radicals (CH3O•) were identified as key C1 intermediates in the photocatalytic oxidation of methane. The self-polymerization reaction of gas-phase methyl radicals led to the production of ethane, indicating the critical role of methyl radical detachment in high-selectivity ethane synthesis. Based on the observed intermediates, the team clearly revealed the reaction network of methyl radicals in methane photocatalytic oxidation, which holds important significance for studying the process of methane photocatalytic conversion.
This work innovatively developed an in-situ photocatalytic mass spectrometry detection technique based on synchrotron radiation, which allows for the capture of gas-phase active intermediates and elucidates the mechanism of methane photocatalytic conversion reactions. The research also holds broad application prospects for investigating the mechanisms of other photocatalytic reaction systems.
Paper link: https://doi.org/10.1002/anie.202304352



