Researchers from Tokyo Medical and Dental University (TMDU) find that pre-basophil and mast cell progenitors differentiate into pre-basophils before producing mature basophils in mice
Credit: Inflammation, Infection & Immunity Laboratory, TMDU
Researchers from Tokyo Medical and Dental University (TMDU) find that pre-basophil and mast cell progenitors differentiate into pre-basophils before producing mature basophils in mice
Tokyo, Japan – Big changes often happen in small steps, and it’s not always easy to see how we get from point A to point B. Now, researchers from Japan have found a new step in the process of cell differentiation that leads to the production of fully functional basophils, which are critical cells in the immune system.
In a study published this month in Nature Communications, researchers from Tokyo Medical and Dental University (TMDU) have revealed a previously unknown intermediate step in the differentiation of precursor cells into mature basophils.
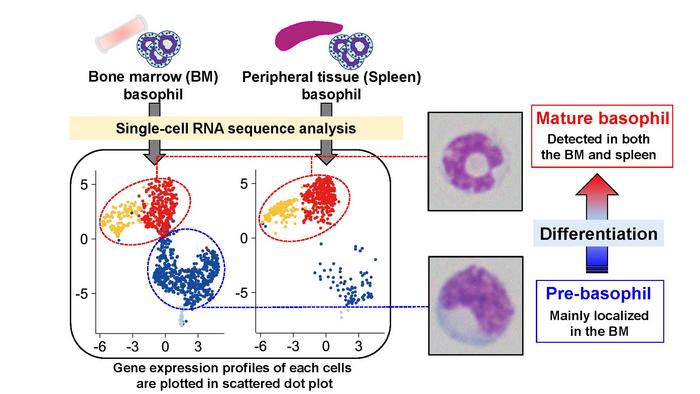
Basophils account for less than 1% of all white blood cells and are involved in allergic reactions and fighting infections. Several progenitor cell populations have been reported to differentiate into mature basophils in the bone marrow, where basophils normally reside until they are needed to respond to an allergen or infection.
“Recent developments in single-cell RNA sequencing (scRNA-seq) technology have challenged the classical understanding of hematopoietic cell differentiation, suggesting that it is a continuous, rather than a stepwise, process,” says lead author of the study Kensuke Miyake. “However, the continuous differentiation trajectory from basophil progenitors to mature basophils remains to be fully explained.”
To determine whether intermediate populations exist between progenitors and mature basophils, the researchers conducted highly sensitive scRNA-seq analysis of basophils derived from mice. The cells were assessed not only for differences in gene expression, but also for changes in protein expression on their surface and for features such as cell size and nucleus shape that characterize different cell populations.
“The results were very striking,” explains Hajime Karasuyama, senior author. “We quickly identified a sub-population of cells with a characteristic surface protein expression profile that corresponded to pre-basophils, a distinct cell population from mature basophils.”
These newly identified pre-basophils showed higher proliferation capacity than mature basophils and were less reactive to IgE (or allergen stimulation) than mature basophils. Unexpectedly, the researchers also found that pre-basophils emerge from the bone marrow to fight parasitic infections in mice and retain their high capacity for proliferation even after having reached the lungs and skin.
“Our findings identify a previously unknown intermediate cell type in basophil differentiation—pre-basophils—which bridges the gap between pre-basophil and mast cell progenitors (pre-BMPs) and mature basophils in mice,” says Dr Miyake.
Intriguingly, an earlier mouse model study identified a similar population of cells that possibly played a role in the regulation of colitis. Given that human basophils express CLEC12A, a protein that is also highly expressed on the surface of pre-basophils in mice, it seems likely that there is a human counterpart of mouse pre-basophils. Further studies are warranted to identify their presence in humans and subsequently gain insights into their role in human autoimmune diseases.
###
The article, “Single cell transcriptomics clarifies the basophil differentiation trajectory and identifies pre-basophils upstream of mature basophils,” was published in Nature Communications at DOI: 10.1038/s41467-023-38356-1
Journal
Nature Communications
DOI
10.1038/s41467-023-38356-1
Article Title
Single cell transcriptomics clarifies the basophil differentiation trajectory and identifies pre-basophils upstream of mature basophils




