A team led by X-ray imaging pioneers at DESY has managed to produce high-resolution images of biological structures at very low X-ray doses. Their new technique was demonstrated by acquiring high resolution X-ray images of dried biological material that was not frozen, coated, or otherwise altered—all with little to no damage to the sample. The procedure uses a type of X-ray interaction commonly used for airport baggage scanning to generate images of the material at nanometre resolution. Using high energy X-rays that are intensely focussed through a set of novel X-ray diffractive lenses, the technique allows imaging to be performed at less than 1% of the X-ray damage threshold of the specimen. The experiment involved researchers from the Center for Free Electron Laser Science, Deutsches Elektronen-Synchrotron, and the Hamburg Centre for Ultrafast Imaging in Hamburg, Germany, and Lund University in Sweden.
Credit: by Tang Li, Lukas Dresselhaus, Nikolay Ivanov, Mauro Prasciolu, Holger Fleckenstein, Oleksander Yefanov, Wenhui Zhang, David Pennicard, Ann-Christin Dippel, Olof Gutowski, Pablo Villanueva-Perez, Henry N. Chapman, Saša Bajt
A team led by X-ray imaging pioneers at DESY has managed to produce high-resolution images of biological structures at very low X-ray doses. Their new technique was demonstrated by acquiring high resolution X-ray images of dried biological material that was not frozen, coated, or otherwise altered—all with little to no damage to the sample. The procedure uses a type of X-ray interaction commonly used for airport baggage scanning to generate images of the material at nanometre resolution. Using high energy X-rays that are intensely focussed through a set of novel X-ray diffractive lenses, the technique allows imaging to be performed at less than 1% of the X-ray damage threshold of the specimen. The experiment involved researchers from the Center for Free Electron Laser Science, Deutsches Elektronen-Synchrotron, and the Hamburg Centre for Ultrafast Imaging in Hamburg, Germany, and Lund University in Sweden.
X-ray light interacts with biological material in a variety of ways, mostly depending on the energy and intensity of the light. At low energies, the X-rays are primarily absorbed by atoms of the sample, which ionise, causing extensive damage to the sample. Images using these low energy X-rays thus map out the sample absorption. At higher energies, absorption is less likely and materials become more transparent. In this case, the process called elastic scattering can be used for forming images, where the X-ray photons “bounce” off of the matter like billiard balls, without depositing any energy. Techniques such as crystallography or ptychography utilise this process. Nevertheless, absorption events still occur, meaning damage to the sample happens anyway. But there is a third interaction: Compton scattering, where the X-rays leave only a fraction of their energy in the target material. This had been ignored as a viable method of X-ray microscopy, since this interaction only becomes dominant at even higher X-ray energies, where no high-resolution lenses previously existed.
The advantage of low dose in the sample using high-energy X-rays poses a challenge to create a lens: such X-rays pass through all materials and are hardly refracted or bent as needed for focusing. A new kind of diffractive lens, called a multilayer Laue lens, was developed to address this. A structure of alternating nanometer-thick layers of silicon carbide and tungsten carbide was first fabricated layer by layer, which was then used to construct a holographic optical element that is thick enough to efficiently focus the beam.
Using this lens system and the P07 beamline at the PETRA III synchrotron light source at DESY, the team imaged a variety of biological materials by detecting Compton scattering as the sample was rastered through the focused beam. This mode of scanning microscopy requires a very bright source, which is focused to a spot that defines the image resolution. PETRA III is one of the only synchrotron radiation facilities which is bright enough at high X-ray energies to be able to acquire images this way in a reasonable time.
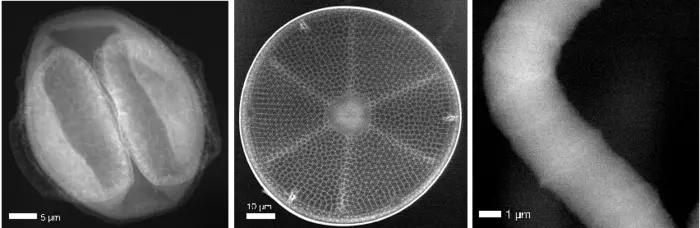
The team used a cyanobacterium, a diatom, and even a pollen grain collected directly outside the lab as their samples, managing a resolution of 70 nanometres. Compared with images obtained from a similar pollen sample using a conventional coherent-scattering imaging method at 17 keV X-ray energy, Compton X-ray microscopy achieved a similar resolution with 2000 times lower dose.
The results of the study showed that the method could be used to obtain images with an even finer resolution of 10 nm before radiation damage becomes a problem. Future upgrades of synchrotron radiation facilities, such as the PETRA IV facility at DESY, would provide the required brightness to achieve this. The method could then be used for imaging whole unsectioned cells or tissue, complementing cryo-electron microscopy and super-resolution optical microscopy, or for tracking nanoparticles within a cell, such as for directly observing drug delivery. The characteristics of Compton scattering makes this method ideal for non-biological uses as well, such as examining the mechanics of battery charging and discharging.
Journal
Light Science & Applications
DOI
10.1038/s41377-023-01176-5