The early stages of embryonic development contain many of life’s mysteries. Unlocking these mysteries can help us better understand early development and birth defects, and help develop new regenerative medicine treatments.
Researchers based at the Australian Regenerative Medicine Institute (ARMI) at Monash University have characterised a critical time in mammalian embryonic development using powerful and innovative imaging techniques, with their work published in Nature Communications.
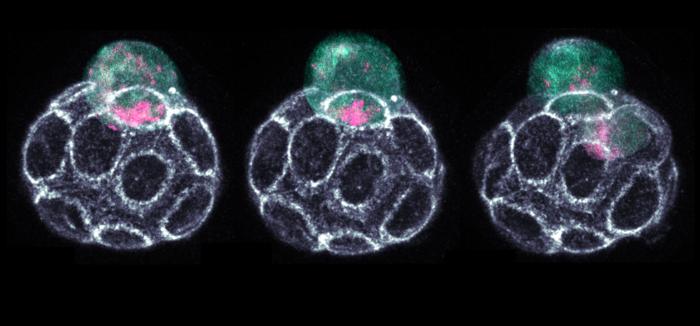
“Just a few days into the journey of embryogenesis, when turning into 16 cells, the embryo must make its first difficult decision – which of its cells will give rise to the embryo or will become extra-embryonic tissue, for example, placenta,” explained lead researcher Dr Jennifer Zenker.
In this study, the research team has discovered how this decision-making process is facilitated by capturing the inner organisation of single cells of the early embryo.
“Ribonucleic acid, RNA, plays a key role here. At the 16-cell stage, the different subtypes of RNA, named rRNAs, mRNAs and tRNAs, are sorted to the two ends of a cell called apical and basal side. The distribution of RNA subtypes determines what the next generation of cells of the embryo will become,” Dr Zenker said.
Interestingly, while most mRNAs and tRNAs remain parked at the apical side, most rRNA molecules travel down to the basal side hitchhiking on organelles called lysosomes. Even though retaining less overall RNA content, the apical sides of outer 16-cell stage cells contain the full collection of RNAs and other factors required for protein production.
Credit: (C) Azelle Hawdon
The early stages of embryonic development contain many of life’s mysteries. Unlocking these mysteries can help us better understand early development and birth defects, and help develop new regenerative medicine treatments.
Researchers based at the Australian Regenerative Medicine Institute (ARMI) at Monash University have characterised a critical time in mammalian embryonic development using powerful and innovative imaging techniques, with their work published in Nature Communications.
“Just a few days into the journey of embryogenesis, when turning into 16 cells, the embryo must make its first difficult decision – which of its cells will give rise to the embryo or will become extra-embryonic tissue, for example, placenta,” explained lead researcher Dr Jennifer Zenker.
In this study, the research team has discovered how this decision-making process is facilitated by capturing the inner organisation of single cells of the early embryo.
“Ribonucleic acid, RNA, plays a key role here. At the 16-cell stage, the different subtypes of RNA, named rRNAs, mRNAs and tRNAs, are sorted to the two ends of a cell called apical and basal side. The distribution of RNA subtypes determines what the next generation of cells of the embryo will become,” Dr Zenker said.
Interestingly, while most mRNAs and tRNAs remain parked at the apical side, most rRNA molecules travel down to the basal side hitchhiking on organelles called lysosomes. Even though retaining less overall RNA content, the apical sides of outer 16-cell stage cells contain the full collection of RNAs and other factors required for protein production.
The crowded basal side, however, is occupied predominantly with rRNAs. Daughter cells obtaining the more active protein factories of the apical side, are more transformable and specialise into the future placenta. The daughter cells which retain their potential to still become any type of cell of the adult organism, called pluripotency, receive the less translationally active bulk of rRNA.
This decision and many like it, which is known as cell fate, are important in development as it determines how these early cells reach their final cell type, such as skin cells, heart muscle cells and brain cells. For regenerative medicine, being able to orchestrate cell fate opens up the capacity to generate new stem cell-based treatments for a number of diseases and conditions.
“As in real life, cells can influence the direction of their own future by getting organised early. Our research may open new ways to predict and direct cell fate decisions,” Dr Zenker said.
Read the full paper in Nature Communications titled: Apicobasal RNA asymmetries regulate cell fate in the early mouse embryo
DOI: 10.1038/s41467-023-38436-2
Journal
Nature Communications
DOI
10.1038/s41467-023-38436-2
Method of Research
Imaging analysis
Subject of Research
Cells
Article Title
Apicobasal RNA asymmetries regulate cell fate in the early mouse embryo
Article Publication Date
30-May-2023




