The Okinawa Institute of Science and Technology (OIST), in collaboration with Astellas Pharma Inc., has developed a new toolkit that uses small molecules to control the activity of a piece of synthetic RNA, and ultimately regulate gene expression. The technology, which was described in the Journal of the American Chemical Society on March 30, 2023, worked in mammalian cell cultures and in mice.
Credit: Y.Yokobayashi/OIST
The Okinawa Institute of Science and Technology (OIST), in collaboration with Astellas Pharma Inc., has developed a new toolkit that uses small molecules to control the activity of a piece of synthetic RNA, and ultimately regulate gene expression. The technology, which was described in the Journal of the American Chemical Society on March 30, 2023, worked in mammalian cell cultures and in mice.
The ability to precisely control whether a gene is turned on or off is expected to lead to more efficient production of compounds that are made using animal cells, and make gene therapy, cell therapy, and regenerative medicine safer.
For genes to be expressed, cells make many RNA copies of a section of DNA. These RNA copies, called transcripts, are then used to make the protein. Scientists can take advantage of this process by introducing additional genes (either as DNA or RNA) into cells, which can then be used to make new proteins for a wide variety of applications. For example, in gene therapy, disorders caused by faulty proteins can be treated by introducing genes into body cells that code for working versions of that protein and in regenerative medicine, the expression of inserted genes could reprogram cells. Proteins with drug applications, like hormones and antibodies, can also be easily produced and collected by inserting genes into cultured cells.
However, after introducing DNA or RNA into cells, scientists need to be able to precisely control the production of the protein, so that it is made at the right time and in the right amount. This maximizes productivity and could help avoid unnecessary side effects in patients.
One method of controlling gene expression is by using ‘riboswitches’. Professor Yohei Yokobayashi, who heads OIST’s Nucleic Acid Chemistry and Engineering Unit and led this research, explained: “This type of technology involves carefully designing an RNA element called an aptamer that can specifically bind to a small molecule. This aptamer is combined with other RNA elements and inserted into the complete mRNA transcript. The mRNA introduced into the cell exists in either an on or off state, depending on whether the small molecule is present, hence the term ‘riboswitch’. This on or off state then determines if the mRNA transcript can be used to make a protein.”
Until now, however, the applications of riboswitches were very limited. The small molecules needed to make the riboswitch active or inactive required high dosages to work, either because they struggled to enter cells, or because they didn’t bind effectively to the RNA aptamer inside the cells, leading to toxic side effects.
“These aptamer sequences are designed and tested with small molecules in a test tube, so designing one that can also work in the complex environment of a cell has proven a major challenge for scientists,” said Prof. Yokobayashi.
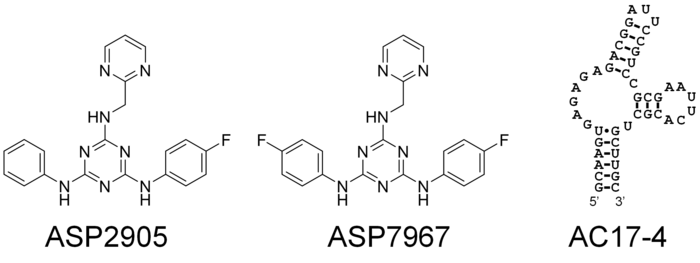
In this study, the researchers selected several small molecules and aimed to develop RNA aptamers that would bind to them inside the cells. The result of their effort was a short RNA sequence, called AC17-4, that binds specifically to two small molecules, “ASP2905” and “ASP7967”, developed by Astellas Pharma. These molecules are drug candidates, so they can be taken up by cells easily and are not highly toxic. ASP2905 was developed for the treatment of neurological diseases and is a drug candidate compound that has undergone its Phase I clinical trial.
The researchers checked whether their riboswitches worked by incorporating them into the RNA transcripts that coded for green fluorescent protein into human-derived, cultured cells. When the two small molecules were administered to the cells, protein production from the RNA transcripts increased 10-300-fold, depending on the design of the riboswitch.
“Only a few riboswitches have shown such an increase in mammalian cells. In addition, using the compounds that are easily taken up by cells allowed the riboswitch to work at concentrations that are less than one-tenth the concentration of previously known riboswitches,” said Prof Yokobayashi.
Next, with gene therapy applications in mind, the researchers at Astellas used a virus to deliver the riboswitch into mice. The riboswitch was designed to regulate the expression of human erythropoietin (hEPO). This protein is used to treat anemia associated with chronic kidney disease and is an attractive target for gene therapy in humans. When the vector was injected into mice, along with oral administration of compound ASP7967, the blood hEPO levels were 7.2 times higher compared to the control group.
Regarding the prospects of this research, Prof. Yokobayashi explained, “The riboswitch developed in this research is already practical enough for non-clinical applications, such as biopharmaceutical production and animal experiments, but further improvement is required for clinical applications, such as gene therapy. We will also continue to research other compounds and aptamers that are under development and showing promising results. In addition, we are considering applying the riboswitch in other organisms, such as plants and insects.”
To achieve these goals, the research team is actively searching for new collaborative partners.
Journal
Journal of the American Chemical Society
DOI
10.1021/jacs.2c12332/
Method of Research
Experimental study
Article Title
Small-Molecule Aptamer for Regulating RNA Functions in Mammalian Cells and Animals
Article Publication Date
30-Mar-2023




