WEST LAFAYETTE, Ind. — A team of Purdue University scientists led by Shihuan Kuang has received a $2.5 million grant from the National Institutes of Health to define the role of lipid droplets in muscle stem cell function, a study with implications in both humans and livestock.
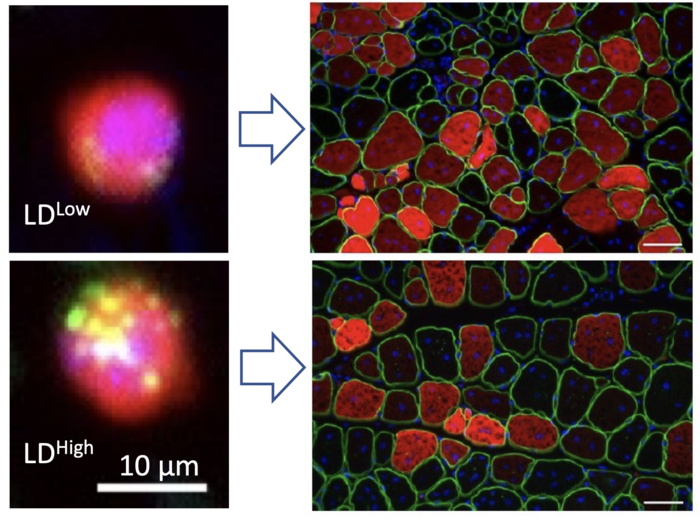
Credit: Image courtesy of Shihuan Kuang, Purdue University
WEST LAFAYETTE, Ind. — A team of Purdue University scientists led by Shihuan Kuang has received a $2.5 million grant from the National Institutes of Health to define the role of lipid droplets in muscle stem cell function, a study with implications in both humans and livestock.
“Lipid droplets are important as a regulatory component of the stem cell. The content of lipid droplets makes the stem cell function better or worse,” said Kuang, Cancer Center Chair in Stem Cell Biology and a professor of Animal Sciences in Purdue’s College of Agriculture.
The human angle will focus on muscle-related disease, aging and injury. The livestock aspect will delve into animal growth to enhance meat production, quality and taste. Working with Kuang on the project are Christina Ferreira, a developmental biologist with training in analytical chemistry in the Bindley Bioscience Center;James Markworth, assistant professor of animal sciences; and Chi Zhang, assistant professor of chemistry in the College of Science.
The grant follows a study that Kuang and eight Purdue co-authors published in the journal Cell Reports last year showing how fat plays an unexpected role in the fate of muscle stem cells.
“We know that if you perturb lipid droplets, the cells do not do well,” Kuang said. “The question now is what do the lipid droplets do in the cell? Do they supplement energy to the cells? Or do the lipid droplets perhaps secrete certain types of molecules that regulate cell function?”
People often think of lipids as bad because they accumulate within the body as fat tissue, Markworth said. The NIH project, however, will explore the potential positive role that lipids may play as important signaling molecules in muscle.
“Right now, we don’t really know what types of lipids are found in these droplets,” he said.
Markworth is especially interested in the role of lipid metabolites – known as bioactive lipid mediators – in muscle biology. Are dietary essential omega-3 or omega-6 (healthy unsaturated fats) found within lipid droplets, for example?
“Does the type of fat that they contain influence their role? And do their various downstream metabolites play different roles in determining stem cell fate?” he said.
In the past, scientists viewed the droplets as inert storage containers, “like a garbage can,” Kuang said. Learning more about how lipid droplets influence stem cells could lead to their manipulation to repair muscle damage more rapidly or to heal muscle disease, he said.
The work has relevance to sarcopenia – common, age-related muscle atrophy – as well as metabolic diseases that affect the muscle, such as obesity and diabetes. The treatment of major genetic muscle diseases, such as Duchenne muscular dystrophy, and of more common exercise-induced and traumatic muscle injury also could benefit.
From an animal science perspective, “lipid droplets are found in the muscle of livestock species, the meat that we eat,” Markworth said. “The composition of lipid droplets in the meat may affect both the taste of the meat and its nutritional value to the human diet. If we can manipulate lipid in the muscle, we could potentially enhance meat quality.”
Markworth joined the Department of Animal Sciences faculty in 2021. His collaborations with Kuang indicate the department’s heightened focus on basic muscle research.
“My lab would like to link the role of lipids and their downstream bioactive molecules in muscle back to lifestyle, nutrition, diet and exercise behaviors,” Markworth said. “If we understand what lipids are important within the cells, their role and how they function, we can manipulate this easily by what kind of lipids we eat.”
Lipid droplets are among many different types of cogs in the cellular machinery. At the Bindley Center’s Metabolite Profiling Facility, Ferreira chemically analyzes the lipids and other small molecules related to the metabolic regulation of the muscle stem cells that the team is studying.
“When cells change their lipid composition, they change their roles in metabolism,” she said.
Ferreira uses an array of highly sensitive techniques, including two developed by Purdue’s Graham Cooks, the Henry Bohn Hass Distinguished Professor of Analytical Chemistry, to profile the metabolism of stem cells and to chemically screen their associated lipid droplets.
“Stem cells are very rare. They appear in small numbers,” Ferreira said. Lipid droplets, meanwhile, are difficult to chemically analyze because of their nanoscale size. Thousands of them could lay side-by-side across the width of a single human hair.
Zhang adds Raman spectroscopy to the project. With this imaging method, he measures the compositions of lipids in live cells. Raman imaging exploits the way that molecular vibrations link to light beams to measure chemical compositions. He also has developed an imaging technique that allows the team to monitor the lipid droplets as the stem cells convert to muscle cells and other cell types.
“We are a group for developing tools. We hope that our tools can be used by biologists,” Zhang said. “Finding applications is always the motivation we have.”
His group currently is designing a tool that can take images of muscle cells while also controlling their fate as they differentiate. The group also is testing how to precisely target a laser only on lipid droplets to see if manipulating the droplets might change the stem-cell differentiation process.
While Purdue’s NIH study will focus on muscle, it has potential implications for stem cells throughout the body, including neural stem cells.
“Lipid droplets might play a similar role in other tissue stem cell types that are not studied yet,” Kuang said.




