Photoelectrochemical (PEC) water splitting presents a promising way to convert solar energy into storable and transportable hydrogen energy. Among investigated semiconducting materials, BiVO4 is considered as an intriguing photoanode candidate, due to its low theoretical onset potential and relatively high photocurrent. Regarding its poor electronic conductivity, Mo doping is demonstrated as an effective strategy for enhancing carrier concentration and n-type conductivity.
Credit: Chinese Journal of Catalysis
Photoelectrochemical (PEC) water splitting presents a promising way to convert solar energy into storable and transportable hydrogen energy. Among investigated semiconducting materials, BiVO4 is considered as an intriguing photoanode candidate, due to its low theoretical onset potential and relatively high photocurrent. Regarding its poor electronic conductivity, Mo doping is demonstrated as an effective strategy for enhancing carrier concentration and n-type conductivity.
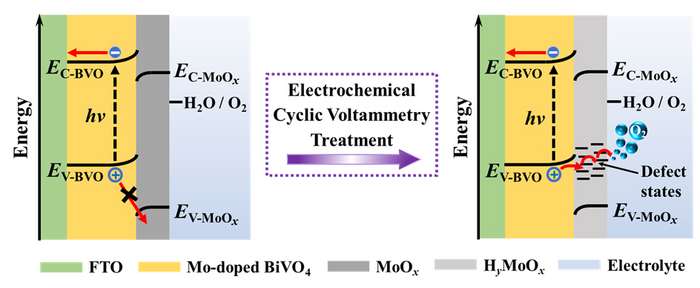
However, MoOx is found to segregate at the surface of Mo-doped BiVO4 photoanodes, which serves as recombination centers and degrades the PEC performance. A previous study has proposed an electrochemical treatment to etch these surface MoOx segregations, thus realizing the activation of Mo-doped BiVO4 electrodes. Yet it is difficult to explain the phenomenon that electrochemically activated Mo-doped BiVO4 electrodes gradually loss their activity when exposed to air at room temperature, because MoOx segregations cannot re-form under this condition. The underlying mechanism for electrochemical activation of Mo-doped BiVO4 needs further clarification.
Recently, a research team led by Prof. Zhaosheng Li from Nanjing University, China offers a new insight into the electrochemical activation of Mo-doped BiVO4 photoanodes: electrochemical treatment not only removes partial MoOx segregations, but also initiates the formation of HyMoOx surface defects which provide charge transfer channels for photogenerated holes. The results were published in Chinese Journal of Catalysis (https://doi.org/10.1016/S1872-2067(21)63986-4).
Using XPS, Raman and XRD measurements, it is revealed that simultaneous reduction and proton insertion in the MoOx species occur during electrochemical treatment via a faradaic reaction: MoOx + yH+ + ye– = HyMoOx. The formed HyMoOx surface defects are sensitive to oxidative environment under which they would be slowly transformed back to MoOx via a deprotonation process: HyMoOx + (y/4)O2 = MoOx + (y/2)H2O. Electrochemical oxidation of one-electron, highly reversible redox couple ferricyanide/ferrocyanide, [FeIII(CN)6]3–/[FeII(CN)6]4–, confirms that MoOx species blocks holes while HyMoOx surface defects act as efficient hole-transfer channels. The energy-diagram change of Mo-doped BiVO4, MoOx and electrolyte system before and after electrochemical treatment suggests that HyMoOx surface defects introduce defect energy levels thereby allow photogenerated holes to transport through.
Based on these experimental results, an explanation to photoactivity variation of electrochemically treated Mo-doped BiVO4 exposing to air is proposed. Upon electrochemical treatment, freshly formed HyMoOx surface defects behave as efficient hole transfer channels thereby increase the photocurrent greatly. When exposing to air, these HyMoOx surface defects would be oxidized and transformed into MoOx, the photoactivity decline depends greatly on environmental temperature and exposure time. Once sufficient oxidation of HyMoOx defects completes, hole-blocking MoOx will dominate at Mo-doped BiVO4 surface and largely suppress the photoactivity.
The introduced concept of surface charge transfer channel is well worth further understanding and optimization, which would offer new opportunities for future development of various fields including energy storage, sensor, and surface/interface science.
###
About the Journal
Chinese Journal of Catalysis is co-sponsored by Dalian Institute of Chemical Physics, Chinese Academy of Sciences and Chinese Chemical Society, and it is currently published by Elsevier group. This monthly journal publishes in English timely contributions of original and rigorously reviewed manuscripts covering all areas of catalysis. The journal publishes Reviews, Accounts, Communications, Articles, Highlights, Perspectives, and Viewpoints of highly scientific values that help understanding and defining of new concepts in both fundamental issues and practical applications of catalysis. Chinese Journal of Catalysis ranks among the top two journals in Applied Chemistry with a current SCI impact factor of 12.92. The Editors-in-Chief are Profs. Can Li and Tao Zhang.
At Elsevier http://www.journals.elsevier.com/chinese-journal-of-catalysis
Manuscript submission https://mc03.manuscriptcentral.com/cjcatal




