When synthesizing new compounds, it is advantageous to have reactants that are widely available and a simple method which produces a high yield of products with little waste (undesirable byproducts). In this regard, the alkylation of alcohols through a method known as “borrowing hydrogen” (BH) can transform alcohols into a diverse range of products, with water as the only byproduct.
Credit: Tokyo Tech
When synthesizing new compounds, it is advantageous to have reactants that are widely available and a simple method which produces a high yield of products with little waste (undesirable byproducts). In this regard, the alkylation of alcohols through a method known as “borrowing hydrogen” (BH) can transform alcohols into a diverse range of products, with water as the only byproduct.
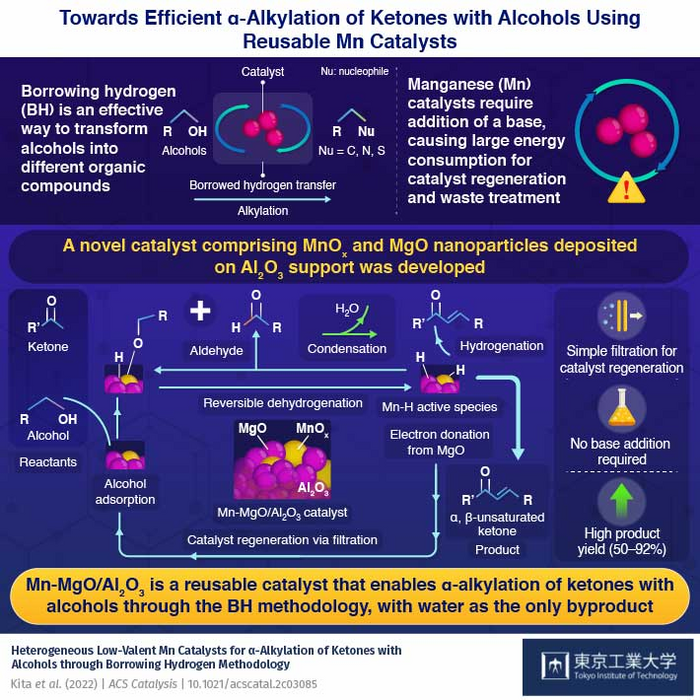
The reaction is centered around a catalyst and begins with a catalyst removing the hydrogen atoms from the alcohol to oxidize it into a carbonyl compound. This highly reactive intermediate then undergoes a condensation reaction with different organic compounds to produce water and receives the hydrogen back from the catalyst to form the final product. Thus, by “borrowing” hydrogen atoms, the alkyl groups of alcohol can be easily transferred to a new organic molecule containing different substituents. Efforts have been made to make this reaction work with inexpensive, bio-compatible manganese (Mn) catalysts. However, currently available Mn catalysts require the addition of strong bases, which generate more waste and complicate the catalyst regeneration process.
Now, in a new study published in ACS catalysis, researchers from Tokyo Institute of Technology (Tokyo Tech) have synthesized a reusable Mn catalyst that can carry out alkylation reactions of alcohols efficiently without the need for a base. The research team, led by Assistant Professor Yusuke Kita, had been previously experimenting with precious metal ruthenium catalysts to carry out the alkylation of alcohols via the BH method. They found that the addition of magnesium oxide (MgO) to the catalyst improved its hydrogenation capability. Wondering if MgO would have the same effect on Mn-based catalysts, they co-deposited Mn oxide (MnOx) and MgO nanoparticles on an aluminum oxide (Al2O3) support.
To their delight, the new catalyst successfully facilitated the alkylation reaction for a wide variety of ketones with alcohols, reaching yields as high as 50–92%. Further, unlike conventional Mn catalysts, it was capable of alkylating ketone-containing substrates, such as cyano groups, which would otherwise react with water in the presence of a base. Most importantly, as the reaction did not produce any byproduct other than water, the catalyst could be easily regenerated with a simple filtration method. “The present catalyst did not require the addition of homogeneous strong bases that are typically indispensable for these reactions and require large energy consumption for separation, recycling, and waste treatment,” says Dr. Kita.
The team attributed the observed high catalytic activity to the close contact between the MnOx and the MgO nanoparticles. Using Fourier transform infrared spectroscopy, a method that can identify chemical compounds by measuring the amount of light they absorb, the researchers identified, for the first time, an Mn hydride (Mn-H) species on the surface of the catalyst, suggesting that MgO facilitates the efficient transfer of hydrogen.
“The catalysis is due to MgO particles that co-exist with the MnOx species in the Al2O3 support and the electron donation from MgO to MnOx enhances the reactivity of Mn−H species,” explains Dr. Kita.
As a cost-effective, easily reusable catalyst, Mn-MgO/Al2O3 could, therefore, accelerate the adoption of alkylation reactions via BH and help produce diverse organic compounds in an environmentally friendly manner.
Journal
ACS Catalysis
DOI
10.1021/acscatal.2c03085
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Heterogeneous Low-Valent Mn Catalysts for α‑Alkylation of Ketones with Alcohols through Borrowing Hydrogen Methodology
Article Publication Date
13-Sep-2022




