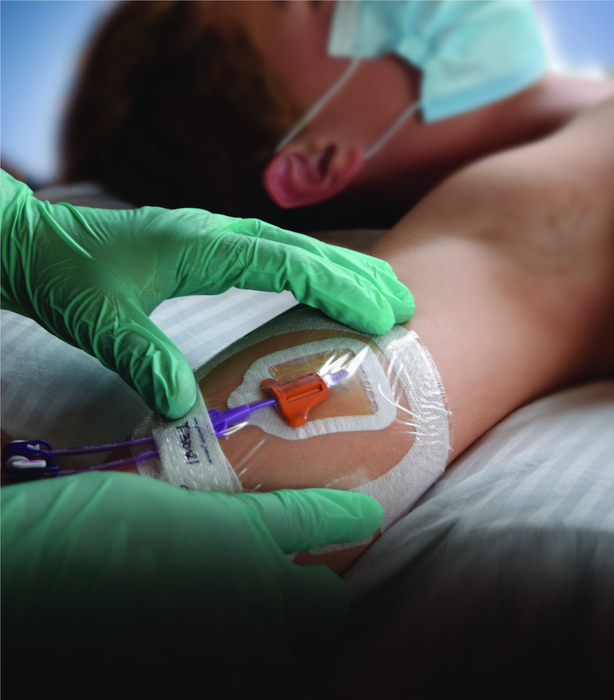
FERNDALE, Mich. — A new study on securement for central vascular access devices (CVAD) suggests that subcutaneous anchor securement systems (SASS) are shown to be more effective at keeping central catheters in place, compared to either suture-based or adhesive device-based securement methods, according to Eloquest Healthcare, Inc., a privately held medical device company.
Credit: Eloquest Healthcare
FERNDALE, Mich. — A new study on securement for central vascular access devices (CVAD) suggests that subcutaneous anchor securement systems (SASS) are shown to be more effective at keeping central catheters in place, compared to either suture-based or adhesive device-based securement methods, according to Eloquest Healthcare, Inc., a privately held medical device company.
Catheter migration and dislodgement have a tremendous impact on patient outcomes, as well as healthcare costs, and “the evidence suggests that subcutaneous anchor systems are superior to other engineered or improvised securement systems when it comes to decreasing catheter migration,” said lead author Jon Bell, MSN, RN, VA-BC.
Published in the Fall 2022 issue of the Journal of the Association for Vascular Access, the review article will also be the subject of an exhibitor theater presentation given by co-author Michelle Hawes, DNP, RN, CRNI, VA-BC at the upcoming annual scientific meeting of the Association for Vascular Access.
A Pressing Problem in Vascular Access
CVADs are essential for patient care, and every month clinicians insert thousands of central venous catheters (CVCs) and peripherally inserted central catheters (PICCs) in patients. Yet there is little conclusive evidence from randomized clinical trials or other studies about which securement methods work best to prevent the catheters from being dislodged or moved, or to reduce bloodstream infections.
This has contributed to “a standard of care that is fractured and confusing and created a real need to gather evidence on the best securement methodology,” said Bell.
Currently, most CVCs are secured using suture-based systems, while the majority of PICCs are secured with combinations of dressings and adhesive securement devices. SecurAcath® by Interrad Medical is the world’s only subcutaneous anchor securement system that can be used on both types of catheters.
Data Show Clear Benefits with Subcutaneous Anchor Securement System
Researchers conducted a systematic review of more than 8,000 studies to examine safety and efficacy outcomes related to CVAD securement. In the studies with good comparative data on rates of catheter migration and dislodgement, researchers found clear benefits for the subcutaneous anchor securement system.
The median incidence of migration and dislodgement for SASS was just 1.76 percent, compared to 4.17 percent for integrated devices (device dressing with built-in securement technology), 6.77 percent for suture-based systems, and 9.69 percent for adhesive securement devices.
The data offered no firm conclusions about which method was best at reducing central-line associated bloodstream infections (CLABSI), and additional research is required to examine the actual effects of securement on patient outcomes. However, Bell suggests that “given the current evidence, it is likely that subcutaneous anchor securement systems are the future of CVAD securement.”
Mounting Evidence for SecurAcath
The new review article adds to the evidence supporting the use of SecurAcath, the only currently available subcutaneous anchor securement system. As Michelle Hawes will describe in her AVA presentation, a 2020 study showed that SecurAcath also significantly reduced the risk of bloodstream infections compared to adhesive securement device methods for PICCs.
Moreover, a retrospective study, found that a single catheter secured with SecurAcath lasted throughout the entire length of need in 98 percent of cases. In contrast, 20 percent of catheters secured with adhesive securement devices needed to be replaced. Despite higher upfront costs with SecurAcath, the better outcomes and simplified care and maintenance will result in lower overall healthcare costs, Hawes will explain.
AVA’s annual scientific meeting will be held September 30-October 3 in Minneapolis, Minn. Hawes’ talk will take place on October 1, 2022, at 12:30 pm CT in Exhibit Hall C of the Minneapolis Conference Center.To learn more about CVAD securement with SecurAcath at AVA, visit Eloquest Healthcare at Booth #612.
About Eloquest Healthcare
Eloquest Healthcare, Inc. is a wholly owned subsidiary of Ferndale Pharma Group, Inc. It is focused specifically on serving hospitals, their healthcare practitioners, and patients. The company has partnered with Interrad Medical to support US sales of the SecurAcath device. Eloquest Healthcare delivers intuitive solutions that complement treatment protocols and address goals related to improving patient outcomes, quality of care delivered, and cost avoidance. More information can be found at www.eloquesthealthcare.com.
Journal
Journal of the Association for Vascular Access
DOI
10.2309/JAVA-D-22-00013
Method of Research
Systematic review
Subject of Research
Not applicable
Article Title
New Study Adds to Evidence on Best CVAD Securement Methodology
Article Publication Date
22-Sep-2022




