The world is highly dependent on fossil fuels to power its industry and transportation. These fossil fuels lead to excessive carbon dioxide emission, which contributes to global warming and ocean acidification. One way to reduce this excessive carbon dioxide emission that is harmful to the environment is through the electroreduction of carbon dioxide into value-added fuels or chemicals using renewable energy. The idea of using this technology to produce methane has attracted wide interest. However, researchers have had limited success in developing efficient catalysts for methane.
Credit: Yang Peng (Soochow University)
The world is highly dependent on fossil fuels to power its industry and transportation. These fossil fuels lead to excessive carbon dioxide emission, which contributes to global warming and ocean acidification. One way to reduce this excessive carbon dioxide emission that is harmful to the environment is through the electroreduction of carbon dioxide into value-added fuels or chemicals using renewable energy. The idea of using this technology to produce methane has attracted wide interest. However, researchers have had limited success in developing efficient catalysts for methane.
A Soochow University research team has now developed a simple strategy for creating cobalt copper alloy catalysts that deliver outstanding methane activity and selectivity in electrocatalytic carbon dioxide reduction. Their research is published in the journal Nano Research on August 01 (DOI 10.1007/s12274-022-4728-1).
Over the past 10 years, scientists have made notable progress in advancing their understanding of catalysts and applying the knowledge to their fabrication. But the catalysts that have been developed have not been satisfactory for use with methane, in terms of selectivity or current density. Despite the great insights scientists have gained, the strategies they have attempted in creating catalysts for methane are just too costly to be useful in practical applications.
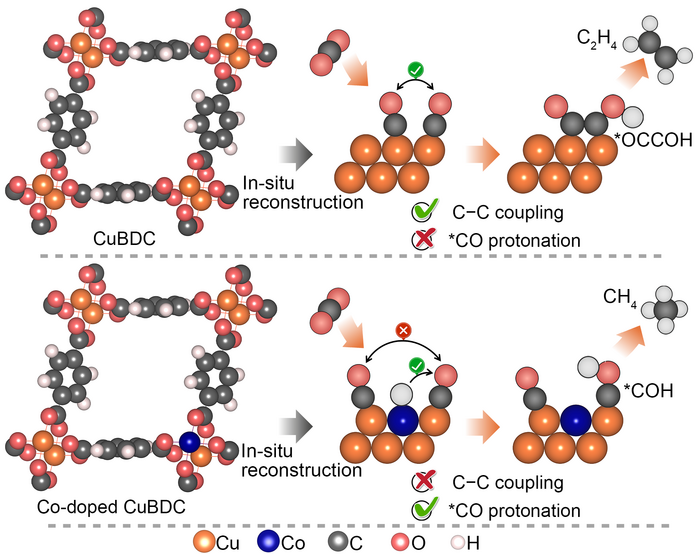
The Soochow University team looked to metal organic frameworks as a way to overcome the earlier challenges in constructing catalysts for methane. “The metal organic frameworks have been perceived as a unique category of electrochemical carbon dioxide reduction reaction catalyst since they offer a tunable platform to systematically alter the metal site coordination, regulate the Helmholtz layer, and control over the intermediates binding,” said Professor Yang Peng, Soochow Institute of Energy and Materials Innovations, College of Energy, Soochow University. The Helmholtz layer refers to the boundary or interface that appears where an electronic conductor comes in contact with an ionic conductor.
Yet the stability of metal organic frameworks during the electrolytic process remains a limiting issue. So metal organic frameworks are often used as the structural precursor to derive more robust catalyst ensembles upon reconstruction. In their research, the team took advantage of the metal organic framework’s homogenously dispersed metal centers. They attained electrochemically reduced cobalt copper alloys that deliver outstanding methane activity and selectivity in electrocatalytic carbon dioxide reduction. The team used in-situ X-ray adsorption spectroscopy and attenuated-total-reflection surface enhanced infrared spectroscopy in the development of their strategy.
The team’s study not only offers a useful strategy for constructing electrocatalytic carbon dioxide reduction catalysts through the electrochemical reconstruction of bimetallic metal organic frameworks, but also furnishes important insights into the steering of electrocatalytic carbon dioxide reduction pathways on copper via atomic doping of 3d transition metals. These 3d transition metals are the elements on the periodic table running from 22Ti to 29Cu (titanium to copper).
By modulating the cobalt doping concentration, the team achieved a remarkable Faradaic efficiency of 60% to methane at a high operating current density.
“The most important message we would like to deliver in this work is that by atomically doping other 3d transition metals in to copper, even in a small quantity, the electrocatalytic carbon dioxide reduction energetics and pathway can be controllably modulated,” said Peng.
As a next step, the team wants to achieve better stability. They will do this by testing the catalytic system in a membrane electrode assembly. “Our ultimate goal is to achieve industrial-scale productivity and stability of methane production and realize the resourceful utilization of carbon dioxide in a green fashion,” said Peng.
The research team includes Hao Sun, Ling Lin, Wei Hua, Xulan Xie, Qiaoqiao Mu, Kun Fen, Jun Zhong, Fenglei Lyu, Zhao Deng, and Yang Peng from Soochow University, Suzhou, China.
The research is funded by National Natural Science Foundation of China, Natural Science Foundation of Jiangsu Province, Six Talent Peaks Project in Jiangsu Province, and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
The paper is also available on SciOpen (https://www.sciopen.com/article/10.1007/s12274-022-4728-1) by Tsinghua University Press.
##
About Nano Research
Nano Research is a peer-reviewed, international and interdisciplinary research journal, publishes all aspects of nano science and technology, featured in rapid review and fast publishing, sponsored by Tsinghua University and the Chinese Chemical Society. It offers readers an attractive mix of authoritative and comprehensive reviews and original cutting-edge research papers. After 15 years of development, it has become one of the most influential academic journals in the nano field. In 2022 InCites Journal Citation Reports, Nano Research has an Impact Factor of 10.269 (9.136, 5 years), the total cites reached 29620, ranking first in China’s international academic journals, and the number of highly cited papers reached 120, ranked among the top 2.8% of over 9000 academic journals.
About SciOpen
SciOpen is a professional open access resource for discovery of scientific and technical content published by the Tsinghua University Press and its publishing partners, providing the scholarly publishing community with innovative technology and market-leading capabilities. SciOpen provides end-to-end services across manuscript submission, peer review, content hosting, analytics, and identity management and expert advice to ensure each journal’s development by offering a range of options across all functions as Journal Layout, Production Services, Editorial Services, Marketing and Promotions, Online Functionality, etc. By digitalizing the publishing process, SciOpen widens the reach, deepens the impact, and accelerates the exchange of ideas.
Journal
Nano Research
DOI
10.1007/s12274-022-4728-1
Article Title
Atomically Dispersed Co-Cu Alloy Reconstructed from Metal-Organic Framework to Promote Electrochemical CO2 Methanation
Article Publication Date
1-Aug-2022




